The importance of Excipient selection for modified release formulations – prevent the risk for alcohol-induced dose dumping

Introduction
The oral route is the main acceptable route, which offers more patient compliance and cost-effective manufacturing processes. Pharmaceutical dosage forms may be developed in which drug release characteristics with respect to time and/or location have in some way been modified compared with conventional dosage forms. These kind of modifications may have several objectives, such as maintaining therapeutic activity for an extended time (better patient compliance), reducing toxic effects, protecting active substances against degradation due to low pH, targeting the active substance to a predefined segment of the gastrointestinal tract for local treatment and product life cycle management for products that are near patent expiry. Thus, modified-release applications continue to be an important development strategy for the global pharmaceutical industry. However, apart from the advantages of modified-release dosage forms, there are numerous challenges in their development. Aqueous solubility, selective stability in the gastrointestinal tract, absorption window of the active ingredients and dose dumping are some of the challenges
in the development of modified-release dosage forms.
Dose dumping is a phenomenon in which a relatively large amount of drug is prematurely released due to an environmental factor and introduced into the systemic circulation. The modified-release formulation contains a higher dose of the drug compared to a conventional dosage form. This may lead to an increase in the plasma concentration of the drug above the therapeutic concentration. In the case of potent drugs, which have a narrow therapeutic index, this may be fatal. In general, dose dumping may be induced by concomitant consumption of food and/or alcohol. Food-induced dose dumping has been well recognized for long decades. In 1985, Leslie Hendeles et al. reported that food-induced dose dumping from a once-a-day Theophylline marketed product ‘Theo – 24’ resulted in a Theophylline toxic effect (1).
Alcohol-induced dose dumping (AIDD) occurs when the administration of a medicine with a modified-release formulation is done simultaneously with the ingestion of alcohol. Although there has been research on the impacts of alcohol on oral modified-release dosage forms for more than a decade, the „Palladone case“ in July 2005 brought AIDD to the attention of regulatory authorities (2). After it has been discovered that serious, possibly lethal, adverse reactions can occur when the extended-release Palladone (Hydromorphone hydrochloride) capsules are taken with alcohol, the US Food and Drug Administration has requested the marketing authorization holder to withdraw the capsules from the market (2).
At the lowest marketed dose of Palladone (12 mg), some patients may experience serious or fatal adverse events; the risk is even higher at the highest strength. A 12-mg Palladone capsule co-ingested with 240 mL (8 ounces) of 40% (80 proof) alcohol led to an average peak Hydromorphone concentration that was about six times higher than when taken with water, according to a pharmacokinetic study in healthy subjects. When the drug was taken in combination with 40% alcohol instead of water, one subject noticed a 16-fold increase in plasma concentration. In some subjects, 8 ounces of 4% alcohol, or about two-thirds of a serving of beer, caused a peak plasma Hydromorphone concentration that was nearly twice as high as when the drug was taken with water (2, 3).
DOWNLOAD FULL MEGGLE WHITEPAPER HERE
Position of Regulatory Agencies on AIDD
Due to concerns about dose dumping of drugs from most of the modified-release dosage forms when taken with alcohol, regulatory agencies currently provide guidance for AIDD in various guidelines in the US, European Union, Canada and Australia. However, the International Conference for Harmonization has not provided any guidance to address AIDD (4).
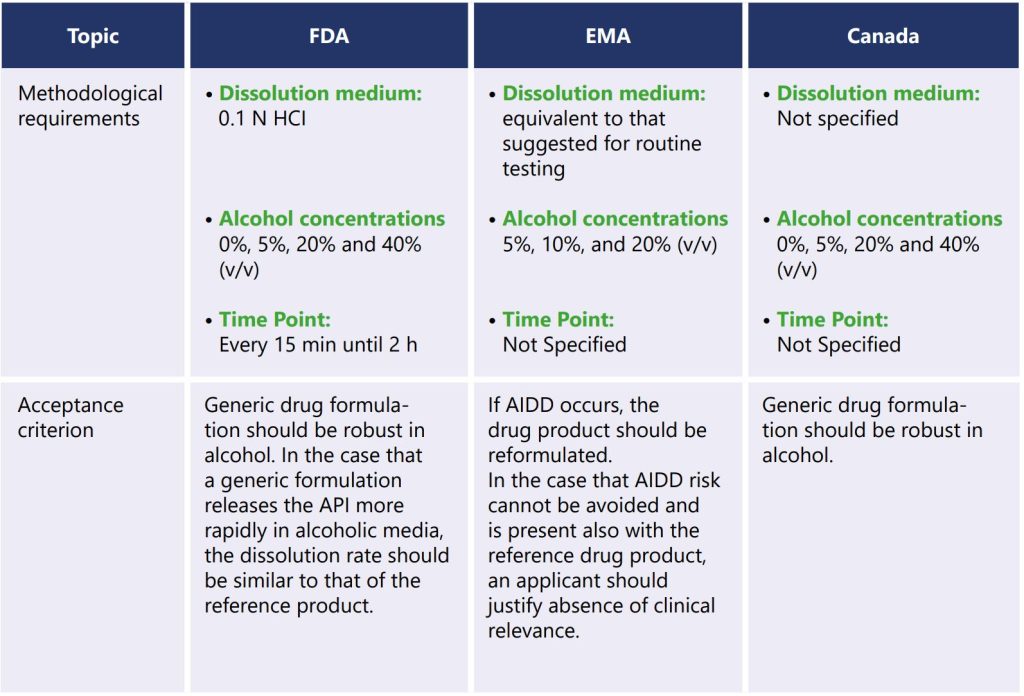
The departments and agencies of the federal government highlighted dose dumping in CFR 21, 320.25(f) (1) (ii). They recommend that the bioavailability profile established for the drug product should obviate the occurrence of any dose dumping. The FDA has provided guidance to the pharmaceutical industry entitled ‘Bioavailability Studies Submitted in NDAs or INDs — General Considerations Guidance for Industry’ which ascertains methodologies for AIDD testing. The FDA recommends that sponsors who are developing certain modified-release oral dosage forms should conduct in vitro assessments of the drug release from the drug product using dissolution media with various alcohol concentrations on the lowest and highest strengths of the modified-release drug product. Based on the findings of this in vitro study, it may be necessary to conduct an in vivo bioavailability study by administrating the drug product with alcohol. The FDA recommends dissolution should be generated on twelve dosage units by using optimal apparatus and agitation speed with suitable dissolution media containing 0%, 5%, 20%, and 40% alcohol. Based on the results of in vitro assessments, an in vivo bioavailability study of the new drug product when administered with alcohol could be needed (5).
The European Medicines Agency (EMA) issued guidance to the marketing authorization holder entitled ‘the pharmacokinetic and clinical evaluation of modified-release dosage forms, which provides recommendations for the AIDD study. EMA recommends AIDD testing be performed by using the same dissolution medium and apparatus as per the validated dissolution method with 0%, 5%, 10% and 20% ethanol. For formulations that release active substances at high or low alcohol concentrations over a short period or at lower alcohol concentrations over a longer period, the formulation should be reformulated. Only in those cases where it can be justified that an in vitro alcohol interaction cannot be avoided by reformulation, an in vivo study could be accepted, in order to validate that such an interaction is unlikely to occur in vivo. The product‘s benefit/risk must be carefully assessed to determine if a significant dose-dumping effect is likely in vivo and cannot be mitigated by reformulation (6).
Health Canada recommends dose dumping should be carried out for opioids and other drug products (e.g., modified-release products) where unintended dose dumping could be potentially fatal to the patient, information on drug release in the presence of alcohol should be provided to demonstrate the absence of dose dumping. Typically, this would involve a one-time dissolution study in an aqueous medium containing ethanol (e.g., release in 5%, 20% and 40% aqueous ethanol solutions) (7).
Although the Therapeutic Goods Administration (TGA – Australia) notified the significance of alcohol dose dumping for modified-release formulations in guidance entitled ‘Assessed listed medicines evidence guidelines’, the testing parameters/conditions are not mentioned in it. However, TGA provided guidance to their market authorization holder in ‘Biopharmaceutic studies, Version 1.2, December 2019’, which defines TGA adopted EMA guideline on the pharmacokinetic and clinical evaluation of modified-release dosage forms; which mentions AIDD testing parameters (8).
The below table describes the requirements of FDA, EMA and Canada regulatory agencies regarding in vitro dissolution testing of formulations at risk for AIDD.
Role of Excipients in AIDD
The key components of matrix tablets affected by hydroethanolic media were identified as wettability, solubility, swellability, and mechanical properties.
Wettability is a fundamental feature of solid-state surfaces that plays a significant role in the modified-release system. The contact angle between solid and liquid interfaces is a common way to describe wettability. The wettability of a polymeric system is an intrinsic property that determines the penetration of solvent into a polymeric system. So, while formulating an alcohol-resilient system, the contact angle should not decrease in the presence of alcohol to prevent an AIDD. Alcohol‘s impact on the release of ionizing and non-ionizing drugs from hydrophilic, lipophilic, and dual matrix tablets was researched by Alena Komersova et al. They have evaluated Hypromellose and Glyceryl behenate as a release controlling matrix system. Glyceryl behenate wettability increases in the presence of an alcoholic solution. Both hydrophilic-lipophilic formulations with ionizing Tramadol hydrochloride and more lipophilic formulations with non-ionizing Pentoxifylline show a significant alcohol dose dumping effect (9).
The solubility of active ingredients and excipients, including polymeric systems, plays a significant role in determining the alcohol resistance of modified-release systems in alcoholic solutions. Typically, polar molecules are more soluble in polar solvents, while non-polar molecules are soluble in non-polar solvents. Moreover, ethanol is less polar compared to pure water, which is reflected by dielectric constants (25 and 80 for ethanol and water at 20 °C, respectively). Tobias Heinrich studied the performance of various oral modified-release dosage forms in hydroethanolic media. This research study concluded that Lactose is a suitable filler in hydrophilic matrix tablets not only for water-soluble Paracetamol but also for water-insoluble Carbamazepine formulations. It enables the drug to be released via diffusion even for poorly soluble drugs and it helps in providing integrity in hydroalcoholic media (10).
Swellability is another phenomenon that affects AIDD. There are two types of polymers that swell in contact with aqueous media:
● Is the so called hydrogels swell in an aqueous system but are insoluble in water.
● the other type is hydrophilic polymers, which swell in the presence of water and are soluble in water.
According to Missaghi et al., the presence of ethanol in the dissolution media may affect the formation of the gel layer and the integrity of the matrix. Roberts et al. observed an initial rapid release of Aspirin from Hypromellose matrices with high standard deviations in 40% ethanolic media. The slower initial interaction between the ethanol and Hypromellose which produces a non-uniform gel layer with inconsistent structure and results in burst release might be attributed to this behaviour (11, 12).
The mechanical strength (crushing strength) of the final dosage form is yet another factor that determines performance in hydro-alcoholic solutions. The hardness of the tablet determines the porosity of the matrix system which affects the penetration of solvent into it. The hardness of tablets mostly depends on active pharmaceutical ingredients, excipients and the manufacturing process. The selection of appropriate manufacturing processes like hot melt extrusion or excipients like co-processed excipients which can offer better crushing strength to the tablet to mitigate AIDD risk (13). A co-processed excipient is any combination of two or more excipients obtained by physical co-processing that does not lead to the formation of covalent bonds. Co-processed excipients have functionalities that are not achievable through simple blending.
Delayed-release formulations combined with the use of alcohol may also result in AIDD. To address growing concerns amongst patients, prescribers and regulatory agencies regarding dose dumping prevention, the excipients industry evoked different novel solutions for retarding drug release in hydro-alcoholic media.
Continue reading the full MEGGLE whitepaper “The importance of Excipient selection for modified release formulations – prevent the risk for alcohol-induced dose dumping” here
DOWNLOAD FULL MEGGLE WHITEPAPER HERE
Authors: Pawan Zade, Prasad Gunjal and Ricarda Leister, MEGGLE Excipients, Wasserburg, Germany
Source: MEGGLE technical brochure The importance of Excipient selection for modified release formulations – prevent the risk for alcohol-induced dose dumping
Do you need more information or a sample of MEGGLE excipients?