Evaluation of Monolayer and Bilayer Buccal Films Containing Metoclopramide
Abstract
Introduction
The administration of drugs via the buccal route has shown benefits, primarily because it allows the drugs to bypass first-pass metabolism and improves the bioavailability of drugs that are highly susceptible to degradation after oral administration [1]. This is a promising route of administration but is unfortunately limited to only specific drugs [2]. These are usually small, potent molecules that exhibit sufficient permeability through the buccal membrane. The application of drugs to the buccal membrane is not always superior compared to oral administration, because of the lower permeability of the mucosal membrane in the oral cavity compared to intestinal mucosa [3]. This type of application might have a great advantage in the case of drugs with poor or variable bioavailability depending on patient metabolism performance [4]. Drugs that undergo extensive first-pass metabolism often exhibit varying concentrations in the blood plasma among patients [5]. The plasma concentrations of drugs play a critical role in determining both their desired therapeutic effects and undesirable side effects. Film formulations used for the buccal administration of drugs have an additional advantage over conventional tablets: they act quickly, resulting in the fast onset of drug action [6,7].
One of the drugs with varying bioavailability is antiemetic metoclopramide (MCP). It is usually administered to oncology patients during chemotherapy to treat nausea and prevent patients from vomiting. Many anticancer drugs have severe side effects such as nausea, so patients need supportive therapy to better withstand the main therapy [8]. MCP is available on the market as a 10 mg tablet, 1 mg/mL oral solution and 10 mg solution for injection. Its variable bioavailability was first reported by Bateman et al., who found that bioavailability in humans varies from 32 to 97% depending on the metabolic performance of the patient [9]. It was later found that the variability was more pronounced at therapeutic doses below 10 mg. Taylor et al. examined the variability of high MCP doses and found that bioavailability was more consistent when patients were given high doses of MCP (2.5 mg/kg) [10]. Nevertheless, the therapeutic doses used are within the range where variability in bioavailability was observed; therefore, the improvement of administration is a possible approach to better therapeutic outcomes.
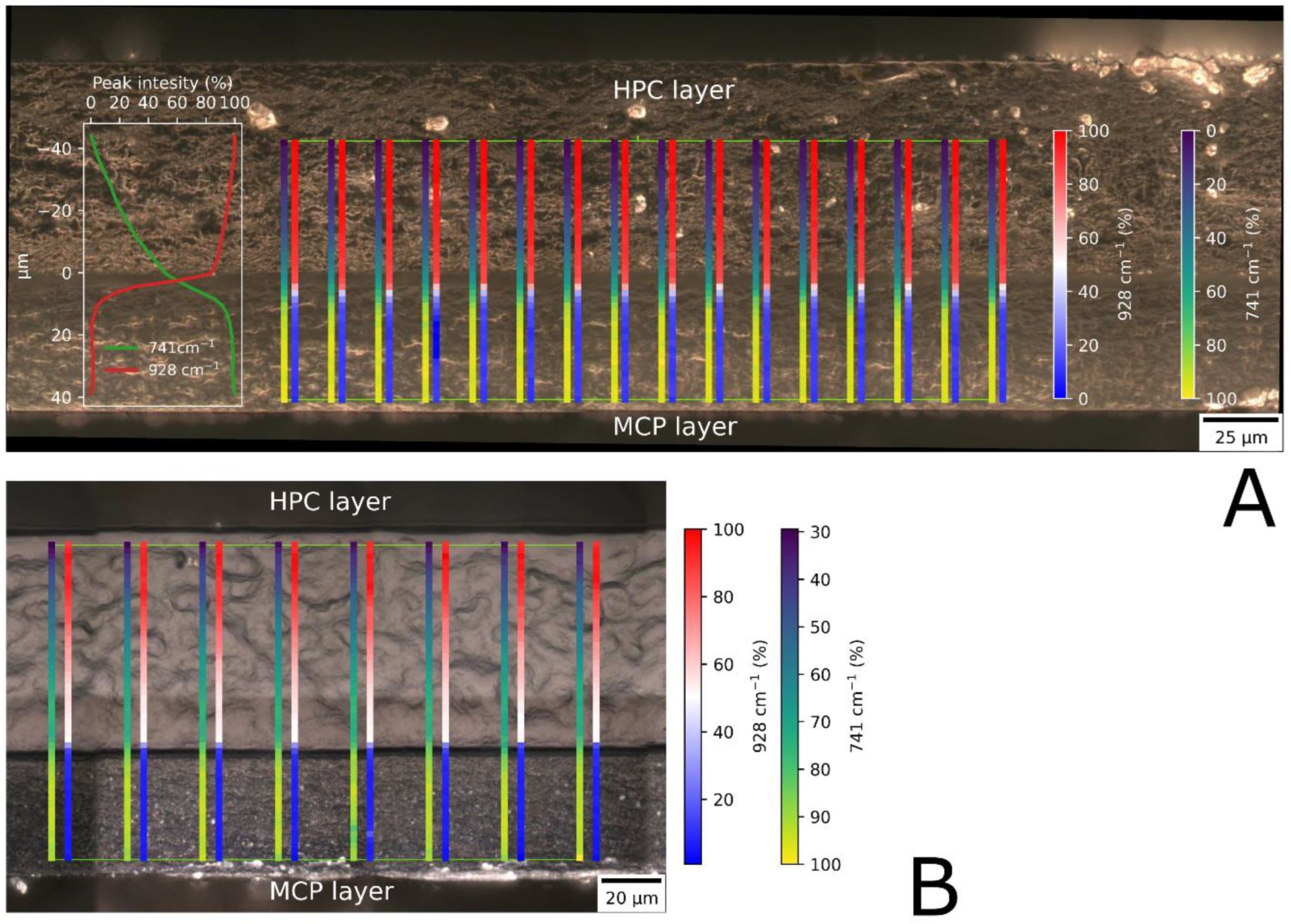
Oral films containing MCP have been presented previously by several authors [11,12,13,14,15,16,17,18]. All the formulations described were prepared as monolayer films in which the active ingredient was homogeneously distributed in the polymer matrix. The drug MCP is freely soluble in water in a salt form and is therefore dissolved in aqueous hydrogels. The high solubility of the drug combined with the bitter taste might not be pleasant for the patient [19]. The contact time of the drug with the membrane is important for adequate absorption by the mucosa [20]. To prevent omnidirectional release of the drug and to prolong the residence time of the film, a second polymeric protective layer could be used [21]. However, the previously presented formulations lacked the additional protective and directing polymer layer.
In sources in the literature, bilayer formulations with unidirectional release can be found [22,23,24,25]. Their advantages are as follows. Double- or bilayered formulations have a different drug release profile, compared to monolayer formulations. The backing layer prolongs the dissolution time significantly [25]. Unidirectional release formulations demonstrate improved permeability when compared to omnidirectional mucosal formulations [26]. It has also been shown that unidirectional release formulations have better permeability compared to omnidirectional mucosal formulations [27]. The protective layer also directs the release of the drug by preventing its release in the oral cavity. It reduces the exposure of the tongue to bad-tasting drugs, which could improve treatment compliance.
The crystallization of drugs from polymer films is a time-dependent phenomenon that is generally undesirable because it can alter the appearance of the formulation and its release performance.
Drugs that crystalize in the formulation may form larger crystals that have a smaller surface area and dissolve more slowly compared with their amorphous analogs [28]. A certain degree of mobility is required for the crystallization of the drug molecule, which is relatively high in formulations such as buccal films [29]. The process of drug crystallization in film formulations should be monitored during the development process. Methods to determine crystallization such as DSC, X-ray diffraction, Raman spectroscopy, etc., are the most suitable but usually destructive or time-consuming. To monitor the crystallization of the film production batch in a more convenient way, a new method would be welcomed. The crystallization of a drug in film formulations might be more easily observed by comparing the visual appearance, since amorphous films are transparent and do not scatter transmitted light [30]. Crystalline film, on the other hand, is opaque and the growth of crystals is usually observed more easily. Therefore, the use of image analysis and computer vision is one way to monitor the crystal growth process [31]. Analyzing images using neural networks trained for this purpose or using a pre-trained network and applying the transfer leaning concept are viable solutions for analysis of images. This was demonstrated by Zupan et al. in the field of medicine by comparing images at different stages of bone healing in mice [32].
The objective of this study was to develop and compare buccal film formulations incorporating MCP. Additionally, these formulations were covered with a backing layer to prolong adhesion to the mucosa and achieve unidirectional release properties. Furthermore, the study aimed to prevent the crystallization of MCP within a Na alginate matrix.
Download the full article as PDF here: Evaluation of Monolayer and Bilayer Buccal Films Containing Metoclopramide
or read it here
Preparation of films
Monolayer formulations
Films consisted of Na-alginate (Protanal 10/60, FMC BioPolymer, Ewing, NJ, USA), xylitol (Xylisorb DC100, Roquette, Lestrem, France) and metoclopramide hydrochloride monohydrate (Biosinth, Bratislava, Slovakia). Formulations were prepared by the solvent casting method. The polymer, plasticizer and the drug were dissolved in water and stirred with a propeller stirrer (Eurostar PWR CV, IKA-Werke, Staufen, Germany) at 1000 rpm until a homogenous solution was obtained. The casting solution pH was adjusted with 0.1 M solution of citric acid to the desired value. Entrapped bubbles were removed by sonication in an ultrasonic bath (1510E-DTH, Bransonic, Eastlake, OH, USA) for 7 min. The degassed casting solution was spread on a glass plate at a thickness of 1500 µm using a motorized coating unit (PIX 1.0, UL FFA, Ljubljana, Slovenia) with an applicator (ZHA 2000.S, Zehntner, Sissach, Switzerland). The films were dried at 60 °C for 60 min in a forced convection dryer (SP −45, Kambič, Semič, Slovenia).
The film composition and pH of the casting solution varied according to the fractional factorial experimental design. The polymer, plasticizer, MCP and pH factors were varied in two levels, which are listed in Table 1. The compositions in bold are repeated experiments at the central point of the experimental design. Factorial analysis was conducted using the Modde 13 analysis tool (Sartorius, Goettingen, Germany). The positive and negative influence of a given factor was calculated using the analysis wizard interface. Square and interaction tests were performed for each measured parameter, i.e., dissolution rate and drug crystallization. The significant factors influencing the measured parameters are presented as the results.
Bilayer Formulations
All formulations from Table 1 were prepared in both a one-layer and two-layer configuration. For the two-layer formulations, a second protective layer was cast on top of the dried drug-containing film. The casting solution for the protective layer consisted of a 20% aqueous solution of hydroxypropyl cellulose (Klucel ELF, Ashland, Covington, KY, USA). The solution was prepared by dispersing the polymer in water heated to 70 °C. While the dispersion was cooled to room temperature, it was continuously mixed with a propeller stirrer. The cooled solution was spread at a thickness of 500 or 1000 µm. The applicator setting for the second layer was adjusted based on the thickness of the first layer to ensure a uniform spreading height of the protective layer. The second layer was then dried at 40 °C for 60 min. After drying, the films were cut into dimensions of 20 × 30 mm and stored individually in airtight primary packaging to preserve their integrity. Bilayered formulations were labeled with an additional letter, “D” or “E”, depending on the thickness (70 or 110 µm) of the second layer.
Formulations with different thicknesses of the second layer were prepared in order to pre-test their influence on the release rate of the drug. An M8 formulation was used as the first layer onto which the HPC layer was casted at 500, 1000 and 1500 um. The dried thicknesses of the HPC layers were 70, 110 and 240 µm, respectively.
Grilc, B.; Planinšek, O. Evaluation of Monolayer and Bilayer Buccal Films Containing Metoclopramide. Pharmaceutics 2024, 16, 354. https://doi.org/10.3390/pharmaceutics16030354
See our next webinar and register here:


