Comparison of Mini-Tablets and Pellets as Multiparticulate Drug Delivery Systems for Controlled Drug Release
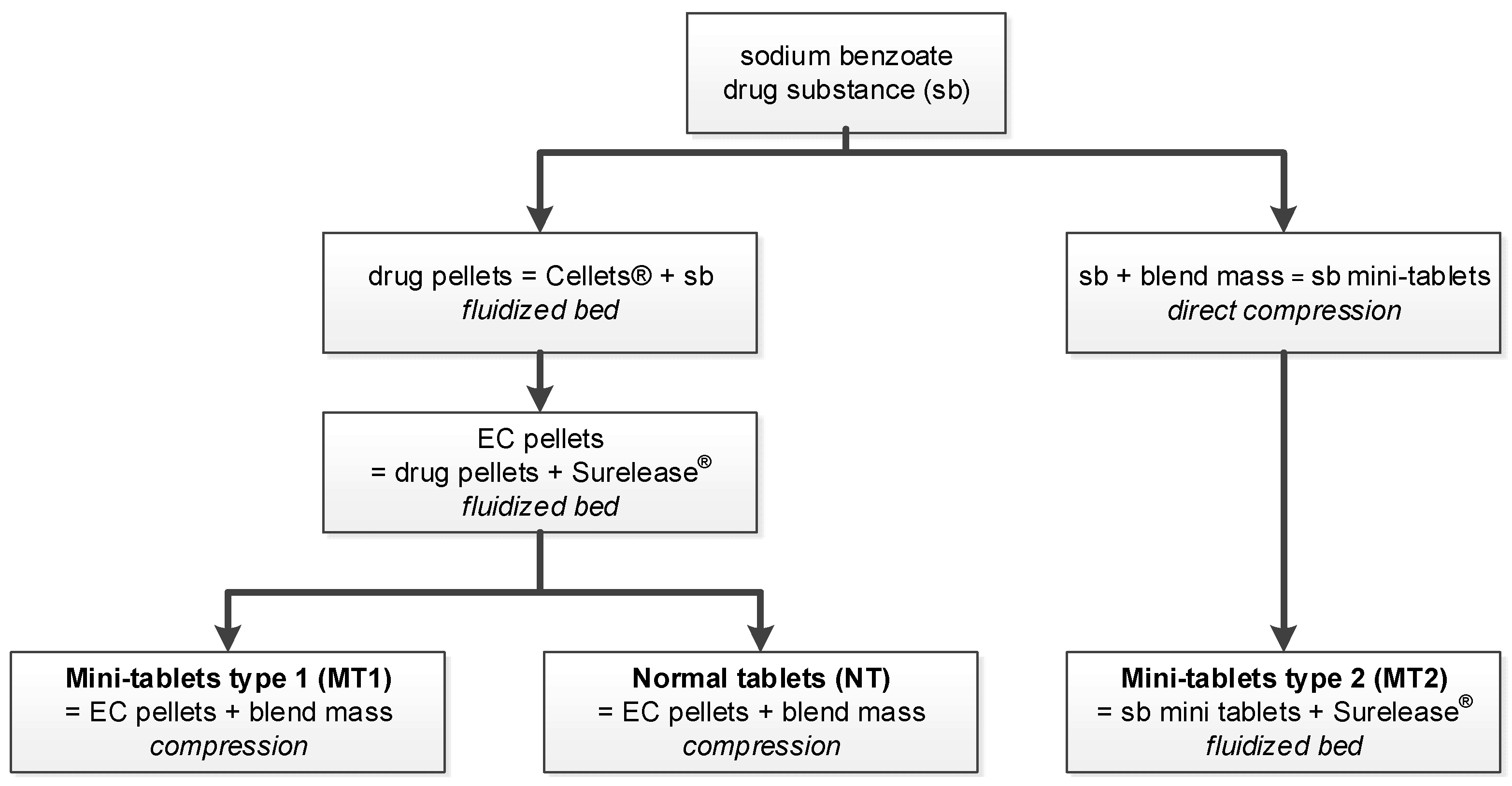
Mini-tablets made into hard capsules or administered using special dosing units, as well as pellets in hard capsules or compressed into tablets, offer the advantages of multiparticulate drug delivery systems and are suitable for controlled drug release using polymer coatings. Four different kinds of solid drug preparations were manufactured and investigated concerning drug release. Inert pellets were coated with the model drug sodium benzoate and, in a second step, with the insoluble polymer ethylcellulose. The coated pellets were compressed into mini-tablets and into normal tablets. Another kind of mini-tablet was compressed from a sodium benzoate compression mixture and finally coated with ethylcellulose. The coating of the tablets was performed using fluidized bed technology.
The sodium benzoate release plots of the coated pellets show a lag time and retarded release according first-order kinetics. The mini-tablets and normal tablets compressed from pellets release sodium benzoate according to first-order kinetics as well, but without the lag time due to distinct ethylcellulose layer destruction during tableting. The release is retarded with increasing ethylcellulose layer thickness on directly compressed mini-tablets. The different formulations of coated pellets, mini-tablets, and normal tablets offer a broad choice for variable drug release kinetics depending on the biopharmaceutical and pharmacological requirements.
Download the full article as PDf here: Comparison of Mini-Tablets and Pellets as Multiparticulate Drug Delivery Systems for Controlled Drug Release
or read it here
Materials
All excipients were used as received. The quality of all substances refers to pharmacopoeia [Ph. Eur. 2020]. Inert microcrystalline cellulose pellets (Cellets®200, IPC, Dresden, Germany), sodium benzoate (Chemie Vertrieb, Magdeburg, Germany), polyvinylpyrrolidone (PVP, Kollidon 30®, BASF, Ludwigshafen, Germany), talcum (Chemie Vertrieb, Magdeburg, Germany), ethylcellulose (Surelease®, Colorcon, Dartford, UK), microcrystalline cellulose (MCC, VIVAPUR®102, JRS Pharma, Rosenberg, Germany), lactose monohydrate (Meggle, Wasserburg, Germany), high-dispersed silicon dioxide (Aerosil®200, Evonik Industries, Germany), carboxymethylcellulose, sodium salt, magnesium stearate, and zinc stearate (all substances from Merck, Darmstadt, Germany) were used.
The formulation of the sodium-benzoate-layered and ethylcellulose-coated pellets is summarized in Table 1, and the formulations of the mini-tablets MT1 and MT2 and normal tablets (NT) are presented in Table 2.
Table 1. Formulation of the final pellets coated with sodium benzoate and ethylcellulose.
| Components of Pellet Lot | Content (%) |
|---|---|
| Microcrystalline cellulose (Cellets®200) | 23.2 |
| Sodium benzoate | 30.2 |
| Polyvinylpyrrolidone | 1.6 |
| Talcum | 0.6 |
| Ethylcellulose | 44.4 |
| Total | 100.0 |
Table 2. Formulation mini-tablets MT1 and MT2 and normal tablets NT.
| Components of (Mini) Tablets | MT1/NT Content (%) | MT2 Content (%) |
|---|---|---|
| Microcrystalline cellulose | 36.6 | 38.8 |
| Sodium benzoate | 15.1 | 15.5 |
| Polyvinylpyrrolidone | 0.8 | |
| Talcum | 0.3 | |
| Ethylcellulose | 22.2 | 20.0 |
| Lactose monohydrate | 18.5 | 19.0 |
| Carboxymethylcellulose, sodium salt | 5.0 | 5.2 |
| Magnesium stearate | 1.0 | 1.0 |
| High dispersed silicon dioxide | 0.5 | 0.5 |
| Total | 100.0 | 100.0 |
Priese, F.; Wiegel, D.; Funaro, C.; Mondelli, G.; Wolf, B. Comparison of Mini-Tablets and Pellets as Multiparticulate Drug Delivery Systems for Controlled Drug Release. Coatings 2023, 13, 1891. https://doi.org/10.3390/coatings13111891
Read more on Introduction to Magnesium Stearate as a pharmaceutical excipient here:


