Towards the development of a biorelevant in vitro method for the prediction of nanoemulsion stability on the ocular surface
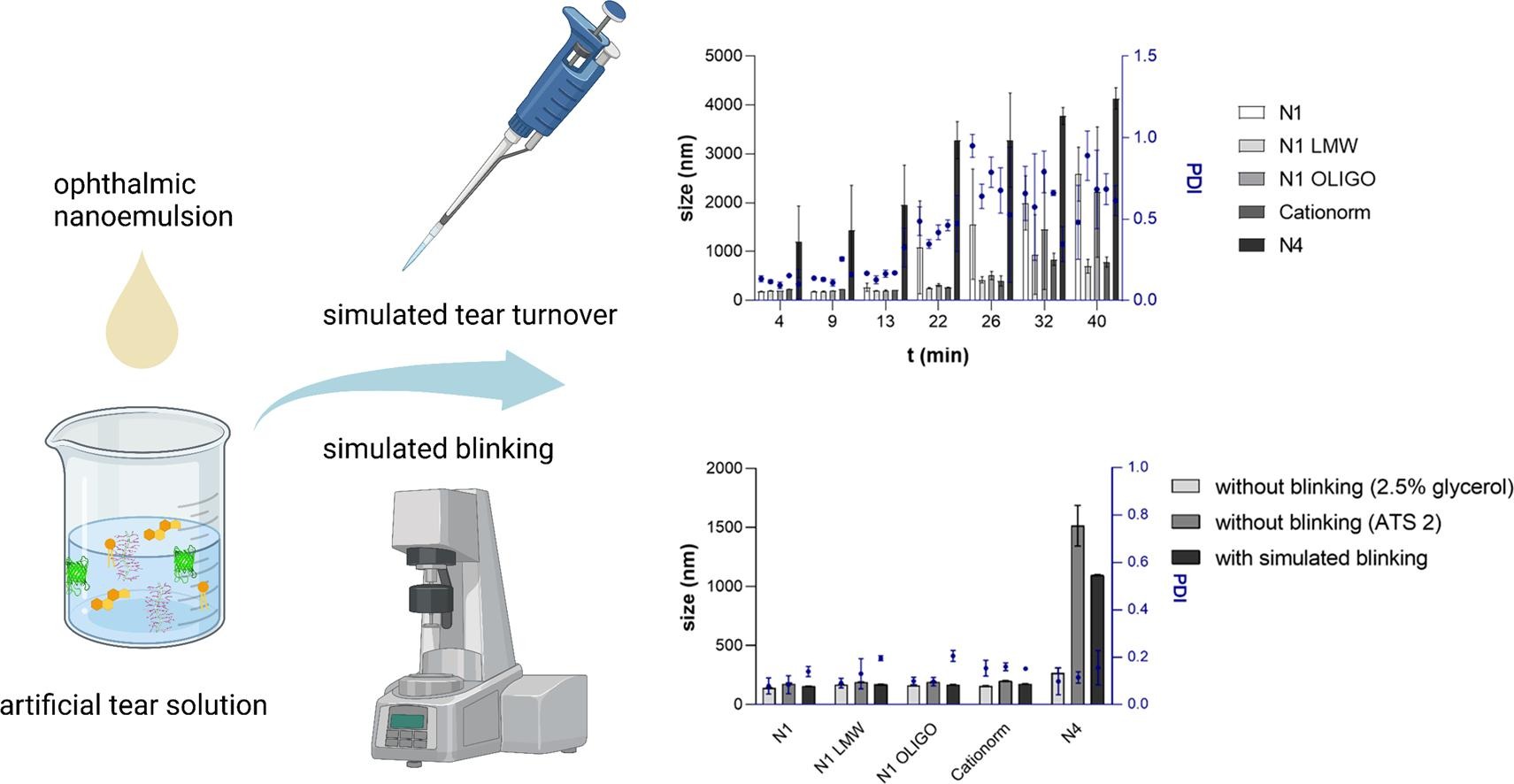
Ophthalmic oil-in-water nanoemulsions (NEs) are a complex technological platform, representing an advancement in the treatment of dry eye disease. In addition to enabling the incorporation of poorly soluble active pharmaceutical ingredients (APIs), NEs provide prolonged residence time of APIs and other formulation components and consequent replenishment and stabilization of the compromised tear film. Ophthalmic NEs have been on the market for over 20 years, but considering their complexity, as well as the complex nature of the ocular surface, they are still a poorly understood advanced dosage form.
The objective of this study was to develop a biorelevant in vitro method that would be able to predict the behavior of ophthalmic NEs after application. With that goal, NE formulations differing in critical material attributes and critical formulation variables were employed and subjected to simulated tear turnover and blinking. By gradually increasing the complexity of the in vitro method, we were able to detect key parameters influencing NE stability. The undertaken study presents a step forward in the development of in vitro tools that are fundamental to the reliable, cost and time-effective development of innovative and generic topical ophthalmic NEs.
Download the full article as PDF here Towards the development of a biorelevant in vitro method for the prediction of nanoemulsion stability on the ocular surface
or read it here
Materials
For preparation of NEs, the following substances were used: Miglyol® 812 (Fagron, Rotterdam, Netherlands), lecithin (Lipoid S 45, Lipoid, Ludwigshafen, Germany), Kolliphor® EL (BASF, Ludwigshafen, Germany), glycerol (Fagron), low molecular weight (LMw) chitosan (Mw range 50–190 kDa, degree of deacetylation (DD) range 75–85 %; Sigma-Aldrich, Steinheim, Germany), and chitosan oligosaccharide lactate (Mw range 4–6 kDa, DD greater than 90 %; Sigma-Aldrich). Artificial tear solution (ATS 0) was prepared by dissolving sodium citrate dihydrate (0.441 mg mL−1, T.T.T. ltd., Croatia), Na2HPO4·2H2O (4.272 mg mL−1, Fluka Chemie GmbH, Switzerland), KHCO3 (0.3 mg mL−1, VWR International, USA), NaCl (5.26 mg mL−1, Gram-mol, ltd., Zagreb, Croatia), KCl (1.193 mg mL−1, Gram-mol ltd.), CaCl2 (0.0555 mg mL−1, Gram-mol ltd.), Na2CO3 (1.272 mg mL−1, Gram-mol ltd.), HCl (2.6 mg mL−1, Carlo Erba Reagents GmbH, Emmendingen, Germany), d-glucose monohydrate (0.036 mg mL−1, Gram-mol ltd.) and urea (0.072 mg mL−1, Gram-mol ltd.) in double-distilled water (DDW). For preparation of ATS with lipids (ATS 1a), the following lipids were used: triolein (0.015 mg mL−1), phosphatidyl choline (0.0005 mg mL−1), cholesterol (0.0018 mg mL−1), oleic acid (0.0018 mg mL−1), methyl oleate (0.012 mg mL−1) and cholesteryl oleate (0.024 mg mL−1), all purchased from Sigma-Aldrich.
Bisera Jurišić Dukovski, Josip Ljubica, Petra Kocbek, Maša Safundžić Kučuk, Iva Krtalić, Anita Hafner, Ivan Pepić, Jasmina Lovrić, Towards the development of a biorelevant in vitro method for the prediction of nanoemulsion stability on the ocular surface, International Journal of Pharmaceutics, Volume 633, 2023, 122622, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.122622.
Remember World Cancer Day 4th February 2023:
(For more information click on the picture)


