Nanoformulation for Enhanced Drug Delivery and Better Patient Compliance

The increasing occurrence of highly potent or frequent-administration therapies in the drug development pipeline means that the low solubility of drugs is becoming a potential bottleneck for pharmaceutical formulators. This not only makes getting new medicines to market more time-consuming, it also hampers the lives of patients in need. In this case study, Losan Pharma – a contract development and manufacturing organization (CDMO) focusing on improved active pharmaceutical ingredient (API) performance – and BENEO, a multifunctional excipient supplier, pool their collective resources. In doing so, they managed to develop a convenient-to-take once-a-day oral solid dosage form prototype of Dexamethasone, an API that’s practically insoluble in water.
One of the most challenging issues to overcome in terms of getting a new chemical entity (NCE) to market, or reaching its full potential, is solubility. Approximately 60% of potential new products exhibit greater or lesser solubility problems, with some industry experts suggesting that the proportion of poorly soluble drugs in the current pipeline is as high as 90%.1,2 Not only do APIs with solubility issues require more complex preclinical studies and clinical trials, they also present formulators with considerable technical challenges.3 The search for appropriate technologies and
formulation methods to enhance aqueous solubility, permeability and dissolution rate can be problematic; plus, poorly water-soluble drugs often need high doses to reach therapeutic plasma concentrations after oral administration.4
It perhaps comes as no surprise, therefore, that improving drug solubility and, consequently, bioavailability, remains crucial for the entire pharma sector.
Wanted: An Easy-to-use Glucocorticoid
The demand for pharmaceutical substances with improved properties continues to drive progress in the field of outsourced product development. Today, many pharma companies are turning to external specialists that offer formulation know-how, appropriate technologies and available capacity – key requirements to reduce both research
and development (R&D) costs and time to market. To cite a recent example, two Europbased experts in formulation development – Losan Pharma and BENEO – joined forces to design a novel administration format for the poorly soluble glucocorticoid medication: dexamethasone (with Dexamethasonratiopharm® 4 mg as a reference listed drug).
To reach this goal, Losan Pharma applied its long-term expertise in the development of nanoformulations with improved API performance. BENEO supplied the carrier, which needed to both convert the API suspension into a dry format and bring the chosen administration format to fruition; this was a convenient and easy-to-use stickpack
containing biphasic release pellets (Figure 1).
From API to administration:
Optimizing drug delivery Dexamethasone is a synthetic corticosteroid with anti-inflammatory and immunosuppressive properties. It is primarily used to treat various inflammatory and autoimmune conditions, such as allergies, asthma, rheumatoid arthritis and inflammatory bowel disease.5 According to the National Cancer Institute (USA), hexamethasone is also approved in combination with other drugs to treat certain types of cancer, including leukemia, lymphoma and/or multiple myeloma.(6) In recent times, dexamethasone has gained attention for its potential to reduce mortality in hospitalized COVID-19 patients requiring oxygen support or mechanical ventilation.7
“In this project, dexamethasone served as a model drug because it is practically insoluble in water,” said Oliver Luhn, Head of Pharmaceutical Technology at BENEO. Generally, the solubility of an API is a critical factor for drug formulators as, first and foremost, it determines the overall efficacy of the drug. If the API is only partly or not fully dissolved in the gastrointestinal fluids at the site of absorption, the drug can’t properly reach the systemic circulatory pathway and evoke the required pharmacological response.4
Oliver Luhn continues: “When administered in tablets, dexamethasone needs to be taken at a high frequency during the day for most indications. This means that patients need to adhere to a special dosage regime, which can pose a considerable challeng to them. Thus, we were tasked by Losan Pharma to deliver a carrier system that facilitated the development of an alternative oral dosage form with increased API bioavailability and minimized the need for repeated administration throughout the day.”
The developers opted to use stickpacks with dual-release pellets: the overall formulation contained both immediate release (IR) and delayed release (DR) pellets. Although the API released from the IR component reaches the bloodstream quickly after administration, the drug in the DR portion maintains the effective therapeutic dose for a prolonged period of time. This biphasic drug delivery system allows the frequency of dosing to be reduced and contributes to the long-term therapy of chronic disease conditions by maintaining the patient’s drug plasma concentration within its therapeutic range. (8) Matthias Rischer, Director Drug Delivery & Innovation Projects at Losan Pharma, comments:
“We see stickpack technology as a way to facilitate oral intake and increase both application convenience and patient compliance, especially for elderly people and for frequently dosed formulations.”
Matthias Rischer, Director Drug Delivery & Innovation Projects at Losan Pharma
He continues: “Last but not least, drugs in a stickpack provide an easy-to-use administration format as they can be swallowed without water.”
Fast-track Development of an API Nanoformulation
The drug formulation process centered on establishing a stable API nanosuspension. Pharmaceutical nanosuspensions are defined as submicron colloidal dispersions of nanosized drug particles stabilized by surfactants and polymers.9 Generally, creating nanosuspensions offers a way to enhance the solubility of “brick dust” APIs. Reducing the particle size to nanoscale significantly increases their surface area, which results in a much higher dissolution rate.3 This may subsequently contribute to improved bioavailability.
To develop the dexamethasone anosuspension, the researchers turned to rapid API wet ball milling in a dual centrifuge (ZentriMix 380R) (Figure 2). Unlike common separation techniques, dual centrifugation (DC) induces additional rotation of the samples during the spinning process. This causes the strong centrifugal forces inside the vials to change direction continuously; combined with the in situ milling beads, this powerful movement results in very effective milling of the samples and excellent homogenization.10 Additionally, developing nanosuspensions of poorly soluble APIs with DC can be very time saving. Of note, the development of a suitable nanosuspension of a poorly soluble drug is often time consuming as a high number of different combinations/ratios of polymers and surfactants have to be tested for every drug compound by milling; in addition, the particle size distribution and the stability of the agglomerates of each combination have to be measured.10

However, by being able to test 40 small-batch (1 g) samples per DC run, the developers were able to rapidly screen for suitable formulation variables (copolymers and surfactants). Incorporating suitable stabilizers into the nanosuspension helps to prevent particle aggregation and Ostwald ripening (redeposition of small particles onto larger crystals) as they create a protective layer around the particles. This reduces their tendency to grow or coalesce.
The formulation screening process also included the particle size distribution assessment of the samples by laser
diffraction. Afterwards, trial formulations were fine-tuned and scaled-up (2 to 10 g). With the Zeta potential (a measure of the magnitude of the electrostatic or charge repulsion/attraction between particles) in mind, the appearance and bulk stability of selected nanosuspensions were tested after 2 weeks of storage at 2–8 °C.
Moving Towards a Marketable Product
The next step was transferring the resulting nanosuspensions into a dry state to prevent Ostwald ripening.3 This was done by fluid bed layering the nanosuspension onto a carrier system. During this process, carrier particles are fluidized, applied (sprayed on) with a coating fluid (active layer of nanoparticles) and then dried. Small droplets and a low viscosity spray medium are crucial to ensure an even product coating. These “dry” suspensions can then be used to produce solid dosage forms such as tablets, capsules or stickpacks with granulates or pellets.
At this drug development stage, special attention was paid to choosing the most suitable base material. It was crucial for the starter particles to experience as little abrasion in the fluid bed as possible – but to benefit from excellent flowability and ensure smooth processing. The developers tested various different carriers during the trials and finally opted for a sieved grade of BENEO’s isomalt with a bulk density of 820 g/L and a flowability of 27 s/100 g (orifice d= 6 mm) (Figure 3).
“As well as good abrasion resistance, the carrier had to be almost instantly soluble in aqueous media. This is key to ensure rapid dissolution of the drug and fast release of the nanoparticles.”
Dr. Oliver Luhn, BENEO
Critical to this decision was isomalt’s ability to enhance the nanoformulation process. Oliver Luhn from BENEO explains: “As well as good abrasion resistance, the carrier had to be almost instantly soluble in aqueous media. This is key to ensure rapid dissolution of the drug and fast release of the nanoparticles. Also, a highly soluble drug core facilitates particle size measurement as there is no interference with non-soluble carrier particles. Not only is the chosen grade of sieved isomalt suitable for fast formulation screening according to the particle size distribution, it also minimizes electrostatic charging, which can lead to segregation or demixing of the components.”

carrier particles (magnification x 50)
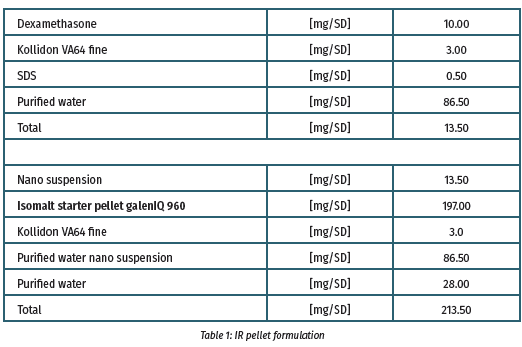
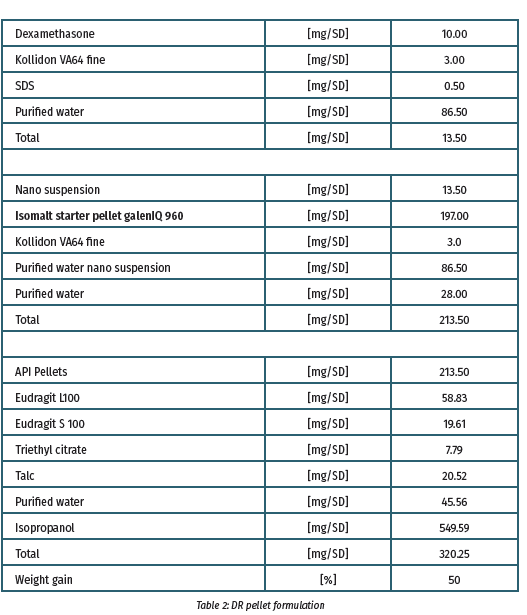
The nanosuspension preparation (batch size 40 g, nanobeads 0.3 mm, 2000 rpm, 90 min, 0°C) was coated onto isomalt particles using the MiniGlatt Micro-Kit with topspray (batch size = 32 g) to prepare the immediate release pellets (Table I). To form the delayed release (DR) components, pellets were covered with a preparation of Eudragit L100 and S100 (3:1) (Table II).
This enteric coating allows the pellets to pass through the stomach and reach the intestine before dissolution begins, thus delaying API release. Finally, IR and DR granules were filled in a 1:1 ratio into a stickpack using a volumetric slider system with two dosing chambers.
Conclusion
During the subsequent in vitro dissolution tests, the IR granules demonstrated complete release of the active ingredient within 10 min (dissolution USP II, 50 rpm, 37 °C, 0.1 M HCI). The modeled dissolution of DR granules showed that release of the second fraction would start about an hour postintake with complete dissolution within the next 2 hours (USP buffer pH 6.8) (Figure 4). Therefore, the developers concluded that the 1:1 filling ratio delivered the desired release profile — immediate release directly after administration and delayed release of the second fraction. Additionally, the obtained results showed no leaking of the modified release pellets. Particle size measurements revealed that the API was maintained in nanoscale during in vitro dissolution and was confirmed by a short-term stability study.
Wolfgang Mohr, Head of R&D at Losan Pharma, says: “We are pleased to note that we were able to achieve a stable nanosuspension of dexamethasone with improved bioavailability using a streamlined production process. The applied nanotechnology offers the advantage of fast set-up and easy integration into a conventional manufacturing line. In addition, using multiparticulate formulations in stickpacks makes a wide range of applications available owing to the different target release profiles (modified, sustained, dual release) that can be achieved by simply changing the filling ratio in the stickpack.”
“With a carrier system that facilitates the development of dosage formats with both immediate and delayed release of the API, it was possible to design a medicinal product that offers extended delivery of the active ingredient in a once-a-day format.”
Dr. Oliver Luhn from BENEO
Oliver Luhn from BENEO adds: “With a carrier system that facilitates the development of dosage formats with both immediate and delayed release of the API, it was possible to design a medicinal product that offers extended delivery of the active ingredient in a once-a-day format. Granules in stickpacks, which can be taken without water, lead to improved compliance, better treatment outcomes and higher patient satisfaction. By diversifying their product portfolio with innovative delivery formats, pharma manufacturers can better cater to different patient needs and gain a competitive edge in the market.
Download the full article as PDF here: Nanoformulation for Enhanced Drug Delivery and Better Patient Compliance
Source: Beneo, www.international-pharma.com,
Author: Dr. Maj-Britt Cepok
Dr. Maj-Britt Cepok is a Food Chemistry graduate and holds a PhD in Analytical Chemistry. She has experience in both the food and pharmaceutical industries, and her particular specialties are the pharmaceutical development of solid dosage forms and taste profiling. Dr. Maj-Britt Cepok joined BENEO in 2005 and has held a variety of roles in global sales, technical services, product management and business development for the Pharma business unit of the company.

