Development of an Oral Liquid Formulation of Nicardipine Hydrochloride Compounded with Simple Excipients for the Treatment of Pediatric Hypertension
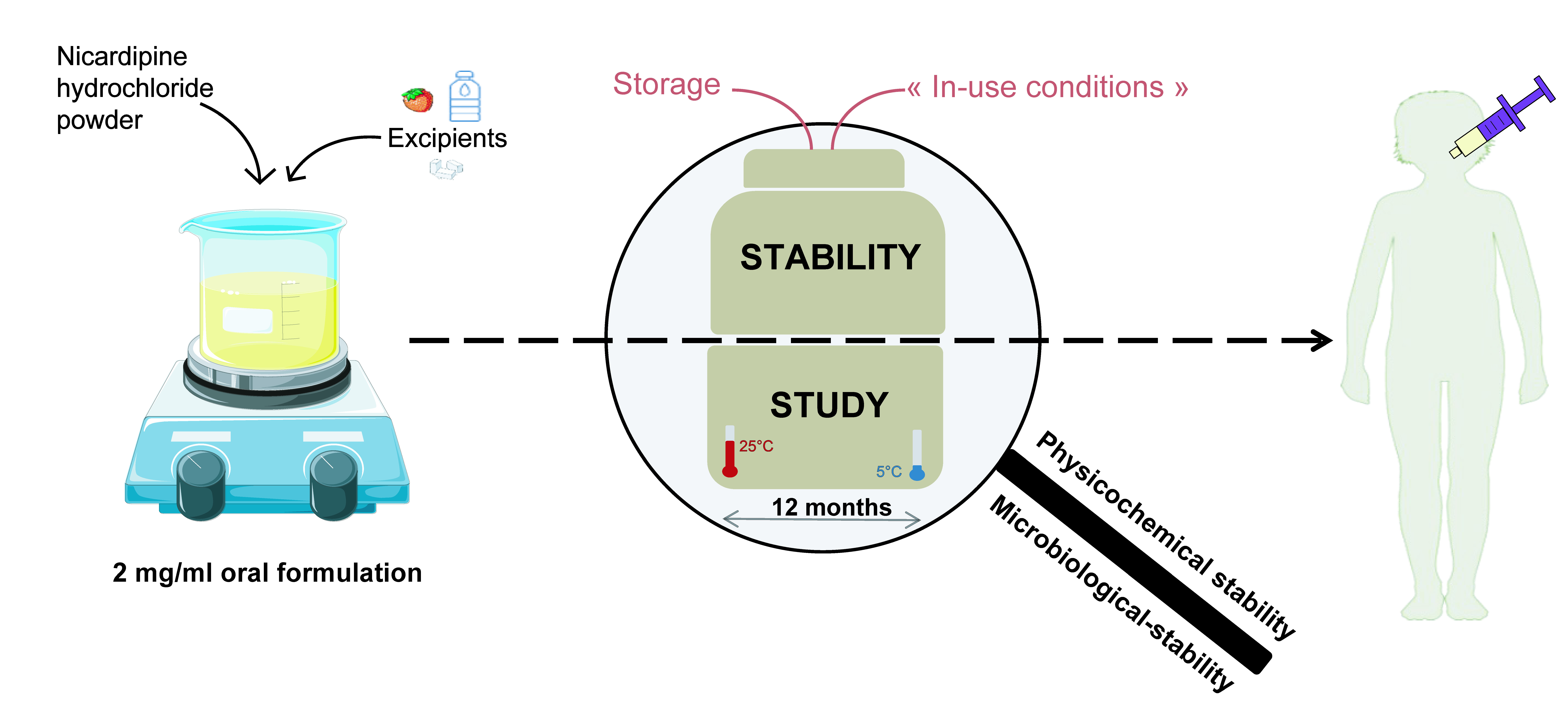
Nicardipine hydrochloride is an anti-hypertensive drug that is used off-label to treat hypertension in children. A previous oral formulation of nicardipine hydrochloride was developed using a commercial vehicle as an excipient. However, ready-to-use vehicles are prone to supply shortages, and their composition may undergo substantial modifications. The aim of this study was to propose a new oral formulation of nicardipine hydrochloride 2 mg/mL using simple excipients. The formulation included hydroxypropylmethylcellulose, simple syrup, polysorbate 80, sodium saccharin, citrate buffer, strawberry flavor and 0.2% potassium sorbate.
The uniformity of content was maintained before and after agitation. Nicardipine hydrochloride concentration assessed by HPLC-MS/MS remained above 90% for 365 days before opening and for 28 days after opening. pH and osmolality were maintained throughout the study, and no microbial contamination was observed. The uniformity of mass of the delivered doses was evaluated using four different devices. A new oral formulation of nicardipine hydrochloride 2 mg/mL was developed using simple and safe excipients. Pharmacological and clinical parameters remain to be assessed and compared with those of the previous formulation.
Download the full article as PDF here Development of an Oral Liquid Formulation of Nicardipine Hydrochloride Compounded with Simple Excipients for the Treatment of Pediatric Hypertension
or read it here
Materials
Nicardipine hydrochloride, polysorbate 80, hydroxypropylmethylcellulose (HPMC) 4000, sorbitol, sodium saccharin and raspberry and strawberry flavors were purchased from Inresa (Bartenheim, France). Citric acid monohydrate, sodium citrate, potassium sorbate, sucrose, glycerol and carboxymethylcellulose were purchased from Cooper (Melun, France) and sterile water (Versylene®) from Fresenius Kabi (Bad Homburg vor der Höhe, Germany).
Reagents were of analytical grade and included acetonitrile, ammonium formate, formic acid, water, methanol, ethanol and sodium hydroxide, purchased from Carlo Erba (Val de Reuil, France). Hydrochloric acid was obtained from VWR Chemicals (Leuven, Belgium) and hydrogen peroxide from Gifrer (Decines Charpieu, France). Nicardipine hydrochloride -d3 was purchased from Toronto Research Chemicals (Toronto, ON, Canada).
Microorganisms (Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 8739), Bacillus subtilis (ATCC 6633), Candida albicans (ATCC 10231) and Aspergillus brasiliensis (ATCC 16404)) were purchased from Bioreference laboratory (Douai, France).
Cavelier, M.; Gondé, H.; Costa, D.; Lamoureux, F.; Pereira, T.; Buchbinder, N.; Varin, R.; Hervouët, C. Development of an Oral Liquid Formulation of Nicardipine Hydrochloride Compounded with Simple Excipients for the Treatment of Pediatric Hypertension. Pharmaceutics 2023, 15, 446. https://doi.org/10.3390/pharmaceutics15020446

