The Nitrosamine “Saga”: Lessons Learned from Five Years of Scrutiny
Abstract
The onset of the N–nitrosamine (NA) saga in 2018 was chiefly related to certain small dialkyl N-nitrosamines originating from the synthesis of the active pharmaceutical ingredient (API). However, the subsequent comprehensive assessments performed on APIs, formulated drug products, and packaging put a different type of NAs in the limelight: a diverse range of complex so-called nitrosamine drug-substance-related impurities (NDSRIs). They may form due to the presence of potentially nitrosatable secondary or tertiary amine moieties in APIs or API impurities and nitrosating agents formed from low levels of nitrite present as impurities. The unique properties of the amine functional group make it irreplaceable in the synthesis of APIs. While nitrite levels may be reduced, the formation of NAs in drug products cannot be completely prevented, and the class default acceptable intake (AI) of 18 ng/day currently poses significant challenges in terms of both viable control and analysis at such low levels.
Even so, NA exposure through pharmaceuticals is expected to be orders of magnitude lower than the exposure via food or endogenous formation. While robust carcinogenicity data are available for many of the small, simple NAs, there is a distinct absence of such data for most NDSRIs. Many working groups have therefore been established to share data and rapidly improve knowledge (whether in terms of toxicity data, structure–activity relationships, or analytical techniques), to define best practices to assess the genotoxic potential of NDSRIs, and to advance methods to calculate AIs based on solid scientific rationales. Ultimately, to protect patients from true cancer risk and secure access to important medicines, it is crucial for manufacturers and health authorities to pursue efforts to implement NA control strategies that are equally effective and realistic. As patient safety is paramount, the pharmaceutical industry is committed to ensuring that the medicines it supplies are safe and effective. Where legitimate safety concerns exist, it is undisputed that appropriate actions must be taken, which could include withdrawal of products from the market.
1. Introduction
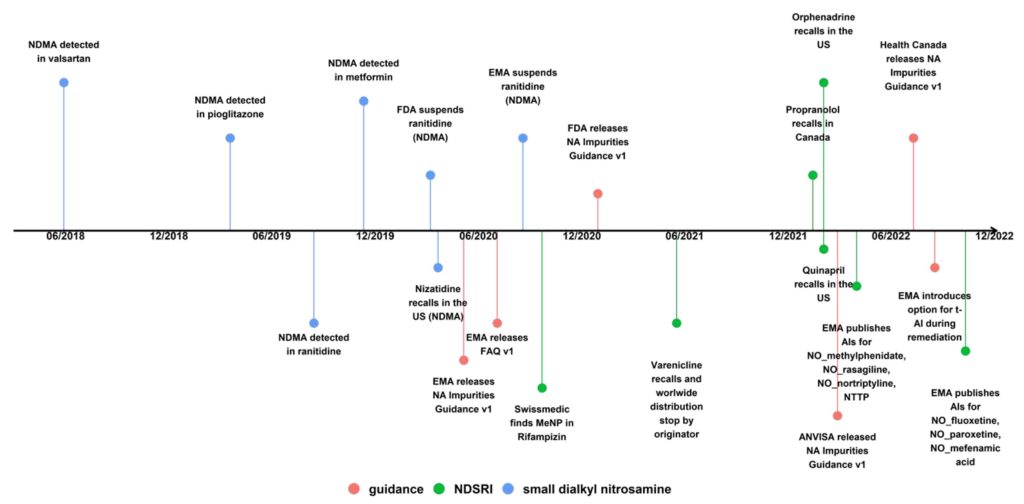
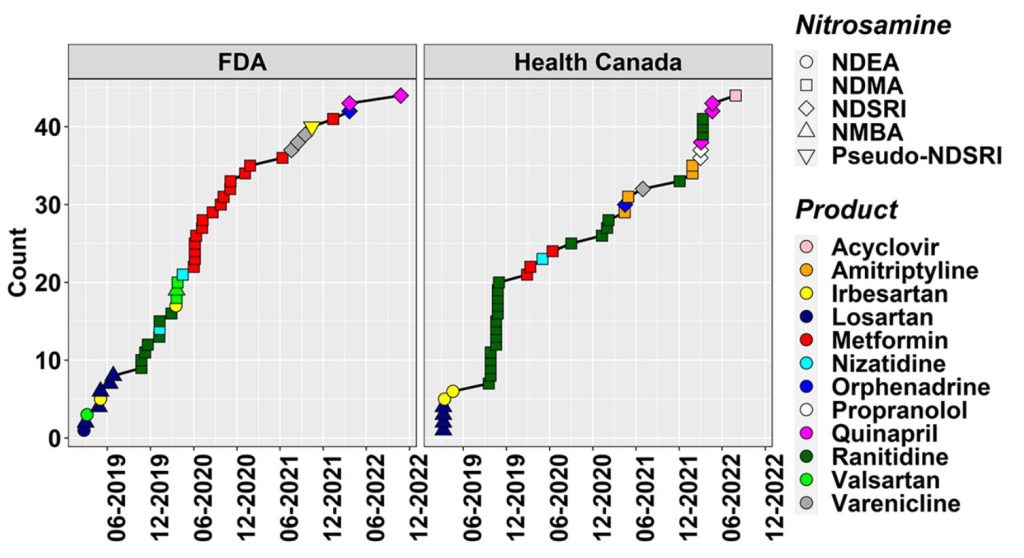
We are already in the fifth year of the N-nitrosamine (NA) saga, which has been a critical concern for pharmaceutical manufacturers and regulators alike since the first detection of N-nitrosodimethylamine (NDMA) in valsartan back in June 2018. NDMA and other small and potent NAs stayed in focus, as they were soon after also found in other sartan active pharmaceutical ingredients (APIs), (1,2) in piaglitazone and ranitidine, (3,4) and in metformin drug products. (5,6) The picture, however, changed dramatically with the recall of Chantix (varenicline) batches in the USA, Canada, and the EU in mid-2021 due to the presence of the nitrosamine drug-substance-related impurity (NDSRI) nitrosovarenicline. (7,8) This was rapidly followed by recalls of propranolol, (9) quinapril, (10) and orphenadrine (11) medicines due to the presence of their respective NDSRIs (Figures 1 and 2). It is to be noted that these recalls were done with the backdrop that there was (and still is) no agreement on how to establish acceptable intakes
(AIs) for NDSRIs , based on read-across or biological tests.
While there is comprehensive literature about the formation of NAs from nitrite in solution (e.g., NAP test), the formation of NDSRIs even in solid drug products (DPs) from parts per million levels of nitrite was surprising. It was recently shown that up to 40% of common APIs and 30% of API impurities are potential NA precursors, as they contain vulnerable amine moieties. If only the more reactive secondary amines are considered, still 13–15% of APIs are potentially at risk. (12) Not surprisingly, NDSRIs have become the focus from both an industry and regulatory perspective.
Industry and regulators are now in the challenging situation that NDSRIs may be present in hundreds if not thousands of medicinal products, some of them affecting whole essential drug classes such as β-blockers. (12) A survey conducted by Medicines for Europe among pharmaceutical manufacturers and presented at the fourth meeting of the EMA Nitrosamine Implementation Oversight Group (NIOG) with Industry Associations on November 30, 2022, revealed that so far 90% of potential NDSRIs identified in NA risk assessments were later confirmed by analytical testing.
This Perspective aims to put the potential risk from NA-containing pharmaceuticals into perspective by reminding us of what is known about NAs from food as well as endogenous NA formation. NAs are formed from vulnerable amines, so we also discuss why these cannot be avoided and, consequently, why pharmaceuticals cannot be made completely NA-free. In addition, we highlight analytical sensitivity challenges arising from the low default AI that is currently applied for some NAs and discuss how more scientifically justified AIs could be derived using read-across. This also examines the potential for the use of AIs in combination with correction for less-than-lifetime exposure and molecular weight. In light of this, the value of in vitro mutagenicity testing (Ames test) and data sharing initiatives remains high. Finally, we summarize major achievements of the past 5 years and give an outlook on the developments we foresee for the nearer future.
2. Nitrosamines from Food
It has been known for many years that there is substantial human exposure to NAs from foods. A recent review (13) highlighted the publication of 122 NA studies covering a range of both NAs and their sources, a significant proportion of which were related to food. The presence of NAs in food was also highlighted in a recent review of NAs by the EMA. (14) Focused on peer-reviewed literature published prior to 2017, the review reported that the food classes with the highest total NA content (TNA) were:
1. Fats, oils, and sweets (average TNA 8.9 ± 3.2 ng/g)
2. Meats (average TNA 8.1 ± 1.4 ng/g)
3. Fish (average TNA 5.6 ± 1.0 ng/g)
4. Vegetables (average TNA 5.4 ± 1.9 ng/g)
Of the NAs, observed the most prominent was found to be NDMA (2.2 ± 0.3 ng/g), suggesting a daily burden in excess of 2 μg/day (based on a 2000 calorie/day diet composed of 500 g/day vegetables, 170 g/day fats, oils and sweets, and 170 g/day meat). A further 1 μg/day exposure may arise from typical consumption of beer or other malt beverages.
As highlighted, the EFSA Panel on Contaminants in the Food Chain (CONTAM) launched a public consultation on the draft scientific opinion on the risks for animal health related to the presence of NAs in food. (14) This document presented an evaluation of the toxicity of NAs, the estimated dietary exposure of European citizens to the carcinogenic NAs present in food, and, based on these, an assessment of the health risks to the EU population. EFSA evaluated 32 NAs and investigated their presence in food. Quantifiable amounts had only been measured for a certain number of the NAs. The risk characterization was therefore limited to the 10 carcinogenic NAs (TCNAs) occurring in food (i.e., NDMA, N-nitrosomethylethylamine (NMEA), N-nitrosodiethylamine (NDEA), N-nitrosodipropylamine (NDPA), N-nitrosodibutylamine (NDBA), N-nitrosomethylaniline (NMA), N-nitrososarcosine (NSAR), N-nitrosomorpholine (NMOR), N-nitrosopiperidine (NPIP), and N-nitrosopyrrolidine (NPYR)).
In total, 2817 results for food samples analyzed from four European countries between 2003 and 2021 were assessed, and in addition, the CONTAM panel also examined results from EU countries (n = 3976) and non-EU countries (n = 27) extracted from articles published between 1990 and 2021, selected based on quality criteria. In comparison to ref (13), the EFSA review adopted a slightly different approach to classification of food categories, defining the five food categories “Alcoholic beverages”, “Coffee, cocoa, tea, and infusions”, “Fish, seafood, amphibians, reptiles, and invertebrates”, “Meat and meat products”, and “Seasoning, sauces, and condiments”. Their evaluation highlighted that in terms of dietary exposure assessment, “Meat and meat products” was the only food category for which data were available for all of the individual TCNAs.
Direct correlation between the two studies is not straightforward. Results of the EFSA review showed TCNAs exposure ranged from 0 to 208.9 ng/kg bw/day across surveys, age groups, and scenarios, with “Meat and meat products” being the main food category contributing to TCNA exposure.
It is interesting to compare exposure levels of nitrosamines from the diet and pharmaceuticals. Focusing specifically on NDMA, the limit for this is defined as 96 ng/day, based on its TD50 value. This level is 10-fold lower than the level of the potent mutagen NDMA typically consumed in food; this is without even considering the benefit associated with pharmaceuticals.
It is important to note that other sources of NAs contribute still further to exogenous exposure, including occupational exposure. (15,16) By far the most significant factor is the use of tobacco products, which truly dwarfs all other exposure sources considering that levels above 20 μg/day have been reported. (13)
Download the full article as PDF here: The Nitrosamine “Saga”: Lessons Learned from Five Years of Scrutiny
or read it here
The Nitrosamine “Saga”: Lessons Learned from Five Years of Scrutiny, Raphael Nudelman, Grace Kocks, Bruno Mouton, David J. Ponting, Joerg Schlingemann*, Stephanie Simon, Graham F. Smith, Andrew Teasdale, and Anne-Laure Werner, Cite this: Org. Process Res. Dev. 2023, Publication Date: July 26, 2023, https://doi.org/10.1021/acs.oprd.3c00100, © 2023 The Authors. Published by American Chemical Society

