Formation of Dialkyl-N-nitrosamines in Aqueous Solution: An Experimental Validation of a Conservative Predictive Model and a Comparison of the Rates of Dialkyl and Trialkylamine Nitrosation
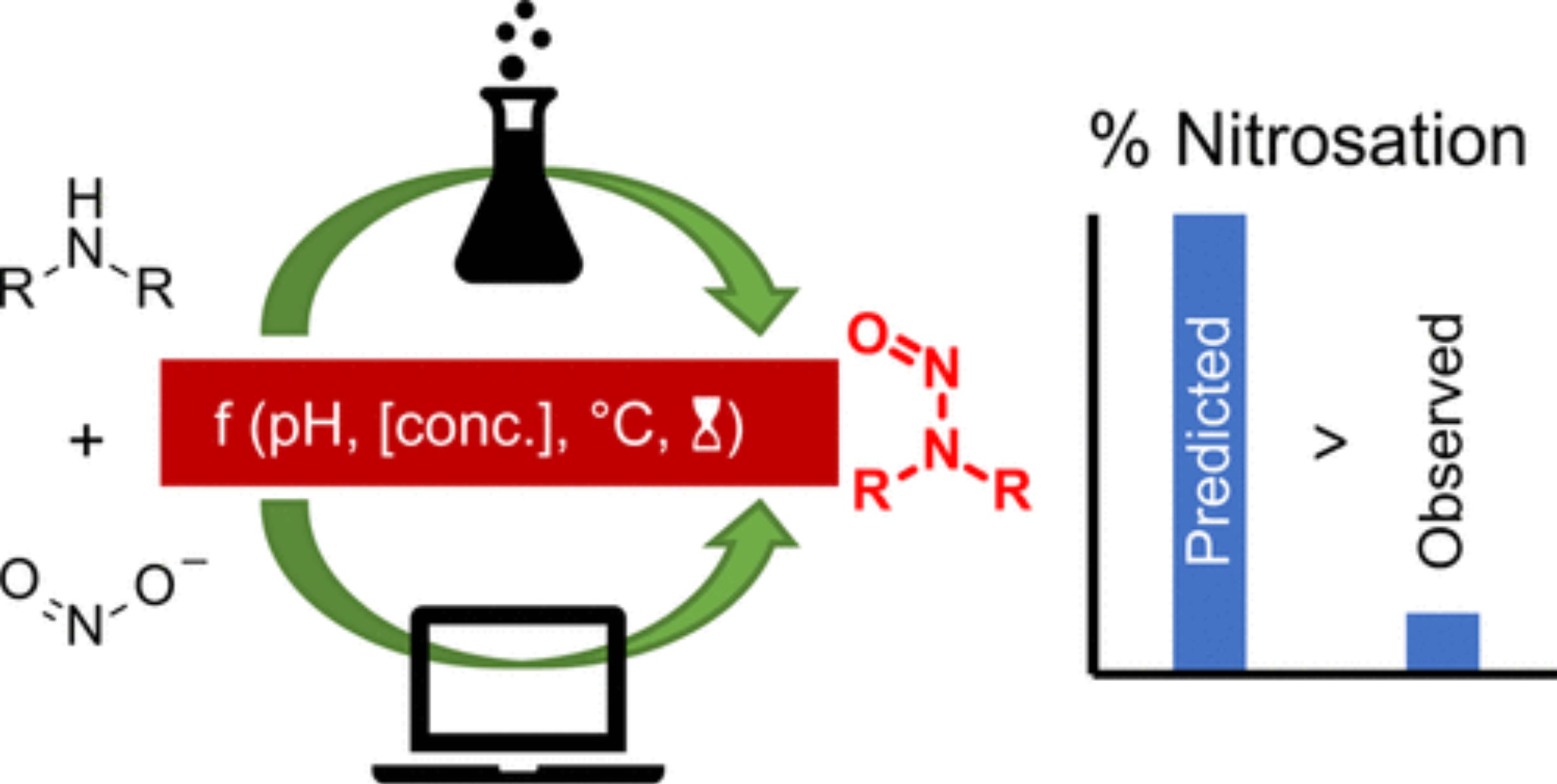
The potential for active pharmaceutical intermediates and active ingredients to contain low levels of N–nitrosamines is a topic of continued interest from industry and regulatory authorities, which has led us to generate experimental data demonstrating that the published kinetic model of dialkylamine nitrosation is conservative and may overpredict the level of N-nitrosamine formation that will actually occur. Additionally, studies comparing the nitrosation of simple trialkylamines to that of the relevant dialkylamines have demonstrated that trialkylamines do indeed undergo nitrosative dealkylation to form N-nitrosamines but at a rate that is at least 500 times lower than for the related dialkylamines.
and download the supporting information here as PDF: Formation of Dialkyl-N-nitrosamines in Aqueous Solution: An Experimental Validation of a Conservative Predictive Model and a Comparison of the Rates of Dialkyl and Trialkylamine Nitrosation
Ian W. Ashworth*, Debasis Dey, Olivier Dirat, Paul McDaid, Daniel Lee, Justin Moser, and Kausik K. Nanda, Formation of Dialkyl-N-nitrosamines in Aqueous Solution: An Experimental Validation of a Conservative Predictive Model and a Comparison of the Rates of Dialkyl and Trialkylamine Nitrosation,
Organic Process Research & Development, Article ASAP
DOI: 10.1021/acs.oprd.2c00366

