Non-invasive peptides delivery using chitosan nanoparticles assembled via scalable microfluidic technology
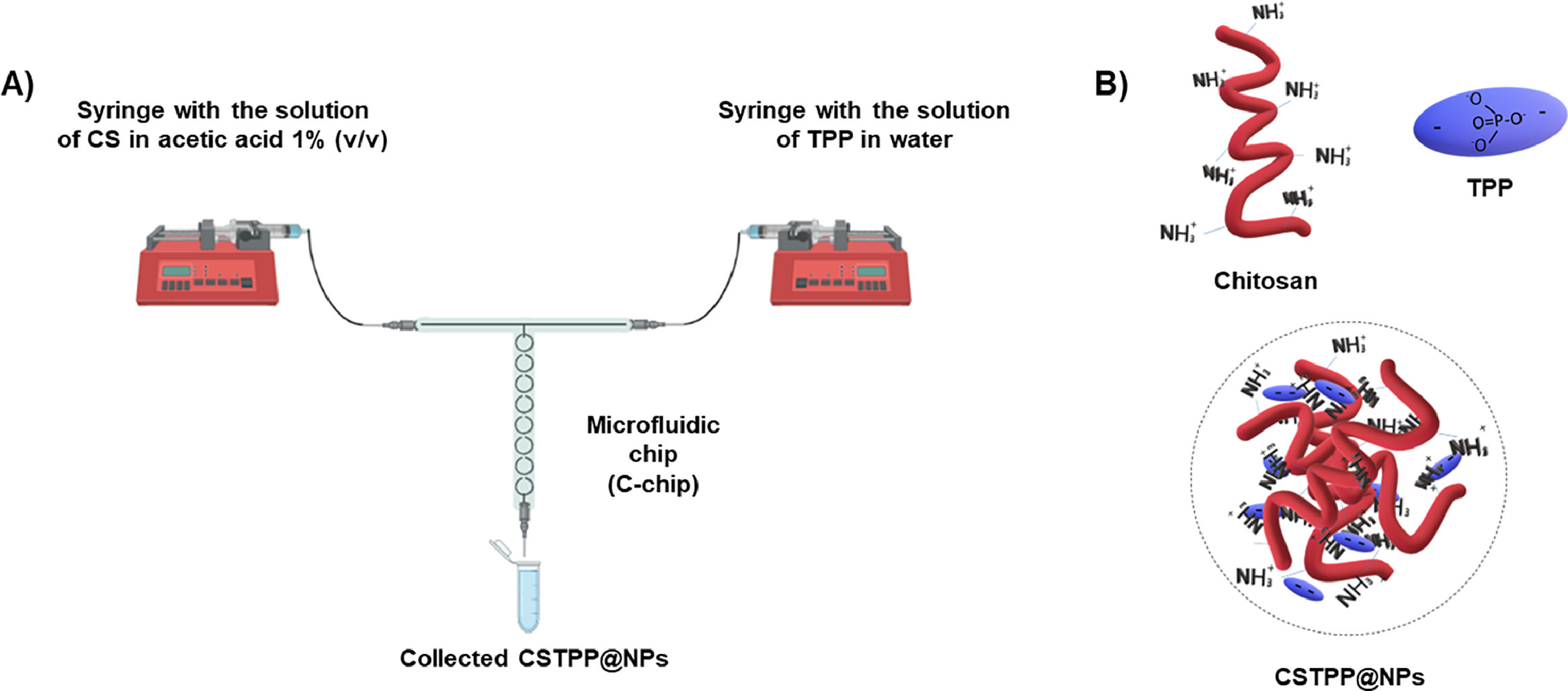
The delivery of peptides via non-invasive administration routes remains a challenge to be addressed. In this regard, chitosan nanoparticles (CS NPs) have shown promise. However, their current batch preparation methods (ionotropic gelation, polyelectrolyte complexing, emulsification solvent diffusion or micro emulsification) have proven difficult to scale up. Here, we established a microfluidic-assisted ionotropic gelation method for the manufacturing of CS NPs, ionically crosslinked with sodium tripolyphosphate (TPP), and loaded with a model peptide, Argireline. The microfluidic process was optimized through a design of experiments approach. CS concentration and pH have the greatest effect on particle size, while CS and TPP concentrations and pH on PDI. The optimum formulation was successfully loaded with the peptide (90% EE) and characterized by a size of 186.0 ± 1.0 nm and a PDI of 0.440 ± 0.002.
Subsequently, Argireline-loaded CS-TPP NPs suspension was converted into a gel for a potential topical application, considering the non-toxic, biocompatible, and biodegradable properties of the components used in the formulation. The NPs gel demonstrated appropriate mechanical properties for Argireline transdermal delivery, along with improved control over its release and enhanced skin permeation for up to 48 hours, compared to NPs suspension and free drug solution. Hence, this study demonstrated that the microfluidic-assisted ionotropic gelation method could be an easy-scalable platform for the manufacturing of peptide-loaded CS-TPP NPs which could be potentially applied for the transdermal delivery of biologics.
Download the full article as PDF here Non-invasive peptides delivery using chitosan nanoparticles assembled via scalable microfluidic technology
or read it here
Materials
Chitosan HCl (Chitoceuticals) with a degree of deacetylation of 82.2 % and a viscosity of 19 mPas (1 % in water, 20 ºC), and an approximate molecular weight of ∼300 kDa was purchased by Heppe Medical Chitosan GMBH (HMC+) (Halle, Germany). Sodium Tripolyphosphate was purchased by Sigma-Aldrich (Sant Louis, USA). Carboxymethylcellulose sodium was provided by Galeno (Prato, Italy). Argireline was kindly donated by Lipotec TM Active Ingredients (Wickliffe, Ohio, USA). All the reagents used were around 96-98% pure. All the solvents used were analytical grade.
Giorgia Maurizii, Sofia Moroni, Javier Vicente Jimènez Núnez, Giulia Curzi, Mattia Tiboni, Annalisa Aluigi, Luca Casettari, Non-invasive peptides delivery using chitosan nanoparticles assembled via scalable microfluidic technology,
Carbohydrate Polymer Technologies and Applications, 2024, 100424, ISSN 2666-8939, https://doi.org/10.1016/j.carpta.2024.100424.

