Investigation of Various Polymers for SLS 3D-Printing of Solid Oral Dosage Forms
Purpose
- Selective laser sintering (SLS) is promising for printing oral dosage forms.
- Print ranges for commonly used pharmaceutical polymers not yet established for additive manufactured medications.
- Evaluate dedicated polymers for pharmaceutical applications.
Objectives
- Determination of optimal print conditions for various pharmaceutical-grade polymers (PVA 4-88 (Parteck® MXP by Merck), PVP-VA1 (Kollidon VA64®), PVP-VA2 (Plasdone™ S-630)).
- Usage conditions of dedicated PVA based polymers P1 (PVA3-82) and P2 (PVA5-74) in SLS printing and impact of hydrolysis degree on printing performance.
Methods
Materials and composition
- 10% API (indomethacin)
- 88.5% polymer (PVA, PVP-VA1, PVP-VA2, P1, and P2)
- 0.5% excipient (silicon dioxide colloidal)
- 1% colorant (silica-based effect pigment)
SLS of dosage forms
- 36 tablet batches created with the same conditions:

- Prints done with three temperatures and three laser scan speeds: 75 °C, 100 °C, & 125 °C and 200 mm/s,
300 mm/s, and 400 mm/s, respectively.
– For some materials, 125 °C was too high, so 112.5 °C was used
– Tablets designed using Fusion360 modelling software:
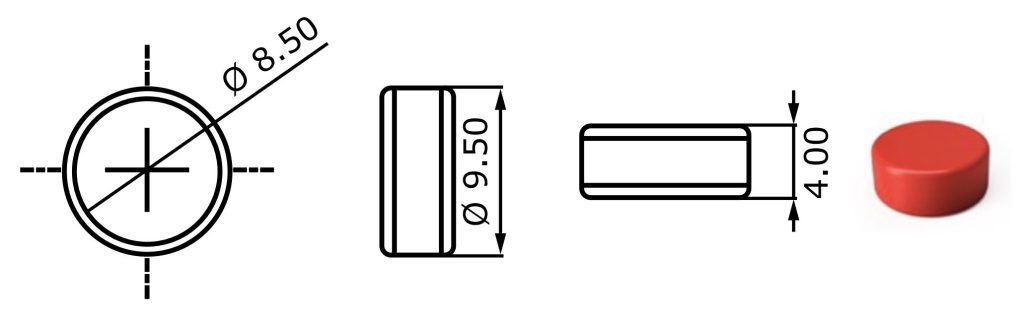
- Printing occurs in layer-by-layer fashion in print bed (tablets fully submerged in powder post-printing, collected via sieving, and dedusted).
- 2.3 W diode (λ=455 nm) laser used.
Characterization
- XRD, DSC, friability, mass and size analysis, HPLC, dissolution.
Results
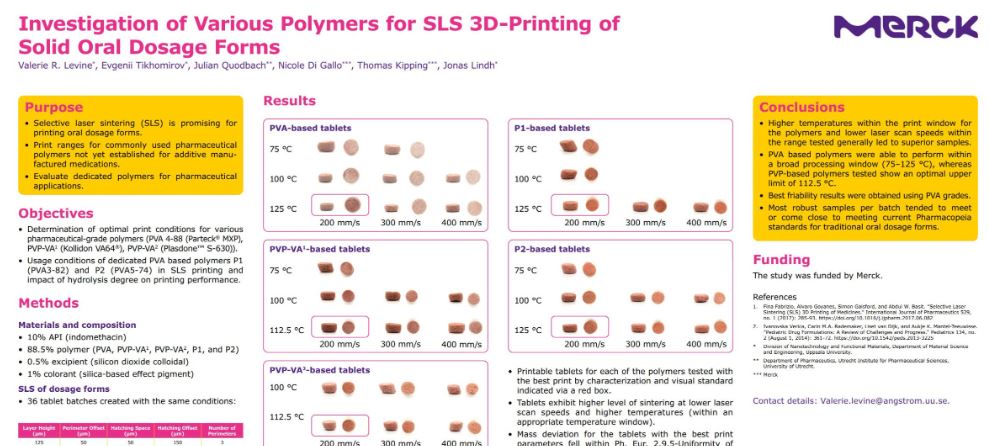
- Evidence of amorphous nature in all of the best print condition samples for each polymer.
- Trends of lower temperature and high laser scan speeds showed more evidence of crystallinity of the API.
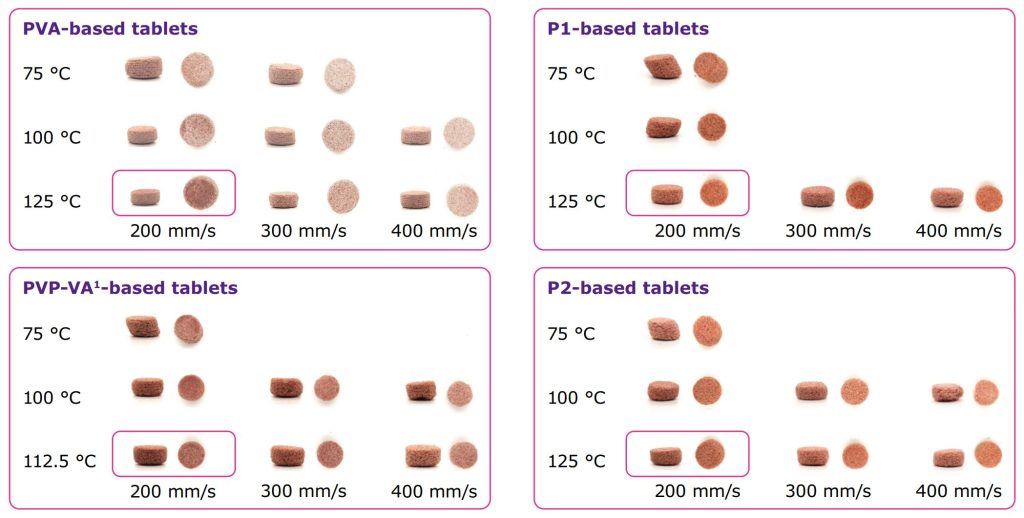
- Printable tablets for each of the polymers tested with the best print by characterization and visual standard indicated via a red box.
- Tablets exhibit higher level of sintering at lower laser scan speeds and higher temperatures (within an appropriate temperature window).
- Mass deviation for the tablets with the best print parameters fell within Ph. Eur. 2.9.5-Uniformity of Mass of Single-Dose Preparation standards for traditional tablets.
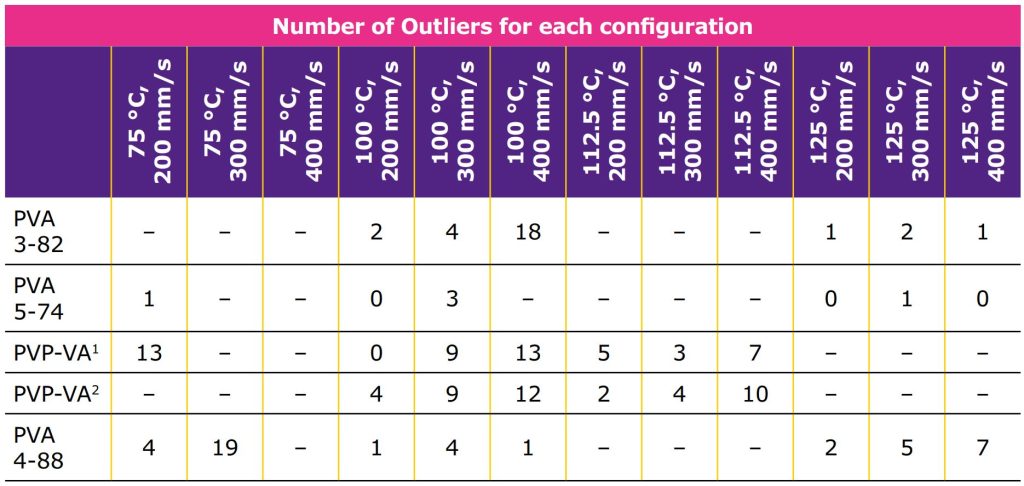
- Number of outliers for tablets with the best print parameters did not always meet Pharmacopeia requirements (<2 outliers), but all viable samples were measured (standards require just 20 at random).
- Friability, while not fully meeting Ph. Eur. criteria for traditional tablets, performed well in some cases, especially for PVA-based tablets.
See the full poster on “Investigation of Various Polymers for SLS 3D-Printing of Solid Oral Dosage Forms” here
(click the picture to enlarge the poster)
Source: Merck technical poster “Investigation of Various Polymers for SLS 3D-Printing of Solid Oral Dosage Forms”
Authors: Valerie R. Levine, Evgeni Tikhomirov, Julian Quodback, Nicole Di Gallo, Thomas Kipping, Jonas Lindh
Do you need more information or a sample of excipients by Merck?


