On the complexity of predicting tablet capping
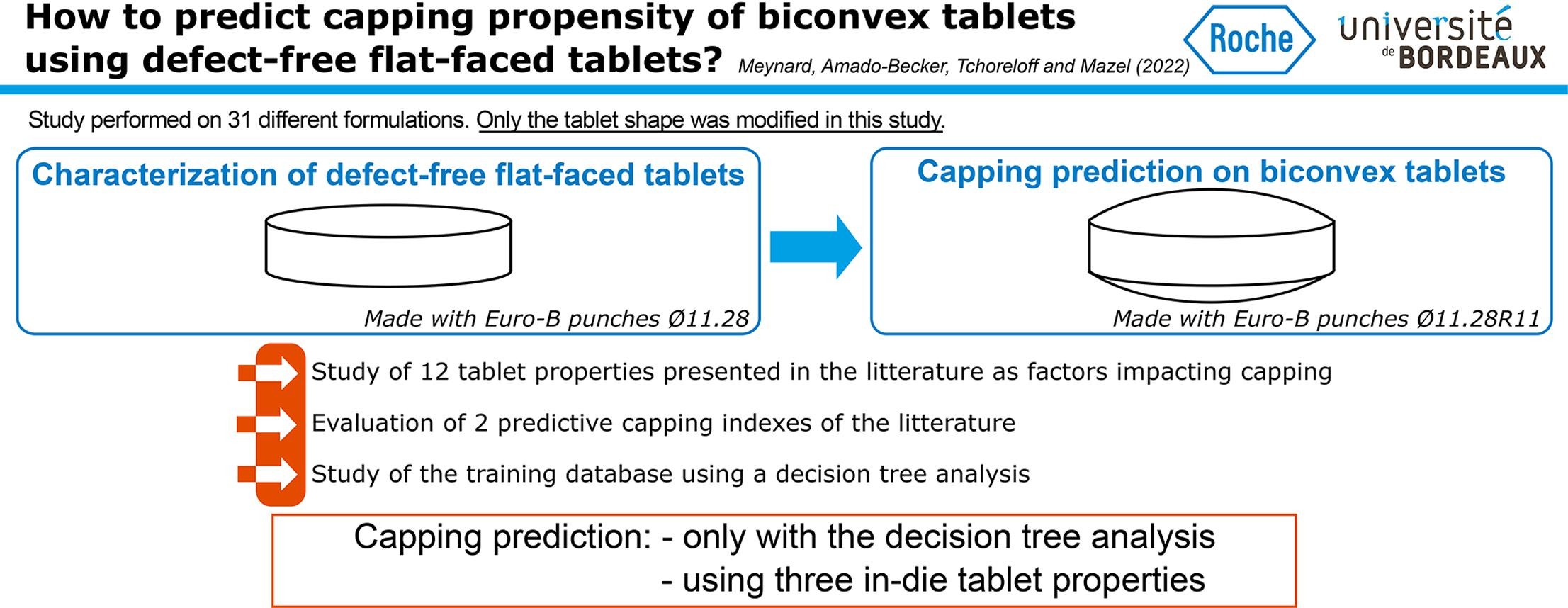
Predicting tablet defects, such as capping, that might occur during manufacturing, is a challenge in the pharmaceutical industry. In the literature, different parameters were presented to predict capping but no general consensus seems to have been reached yet. In this article, we chose to study a wide range of products (18 formulations, 8 of which presenting capping) to predict capping on biconvex tablets using the properties characterized on defect-free flat-faced tablets (tensile strength, solid fraction, elastic recovery, etc.), made using the same process parameters. Single parameters and predictive indices presented in the literature were evaluated on this set of formulations and were found not suitable to predict capping.
A predictive model was then developed using a decision tree analysis and was found to depend only on three in-die tablet properties: the plastic energy per volume, the in-die elastic recovery and the residual die-wall pressure. This model was tested on another set of 13 formulations chosen to challenge it. The capping behavior of 29 out of the 31 formulations studied in total was well estimated using the developed model with only two products which were predicted to cap and did not. This shows the potential of the used approach in terms of risk analysis and assessment for capping occurrence.
J. Meynard, F. Amado-Becker, P. Tchoreloff, V. Mazel,
On the complexity of predicting tablet capping,
International Journal of Pharmaceutics,
Volume 623, 2022, 121949, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2022.121949.
See also our article
10 Errors that can occur during the coating process and how to avoid them


