Preparation and Characterization of an Antiviral Gel
ABSTRACT
The study was to develop an antiviral gel for topical application. The topical application of drug has many advantages over the intravenous and oral administration. It prevents metabolism of drug from liver and avoid the risk of gastrointestinal disorder and inconvenience of intravenous therapy pain. When the drug is applied topically it can penetrate deeper into the skin hence show better absorption and bioavailability. The wide varieties of pharmaceutical dosage form are available for topical drug delivery system. The most used one is gel, ointment and cream. Herpes Simplex Virus (HSV) is widely spread that cause infections. There are two types of this virus, one is HSV-1 usually causes sore around the lip or inside the mouth that are sometime called fever blisters or cold sore and the second is HSV-2, it causes sore on the genitals. Acyclovir is a selective and effective antiviral agent.
Acyclovir remains the gold standard in the treatment of Herpes Simplex Virus infections, mainly due to emerging new drug delivery systems which are improving the bioavailability and fewer side effects. The gel was prepared in different batches or formulation by using different types of polymers in varying concentration viz. Carbopol-934, Carbopol-940, HPMC, and Na-CMC. The gel formulations were evaluated for physical and chemical characterization like visual appearance, drug content, pH, viscosity, extrudability, spread ability etc. and the results was compared.
2.1 Formulation of Gel:
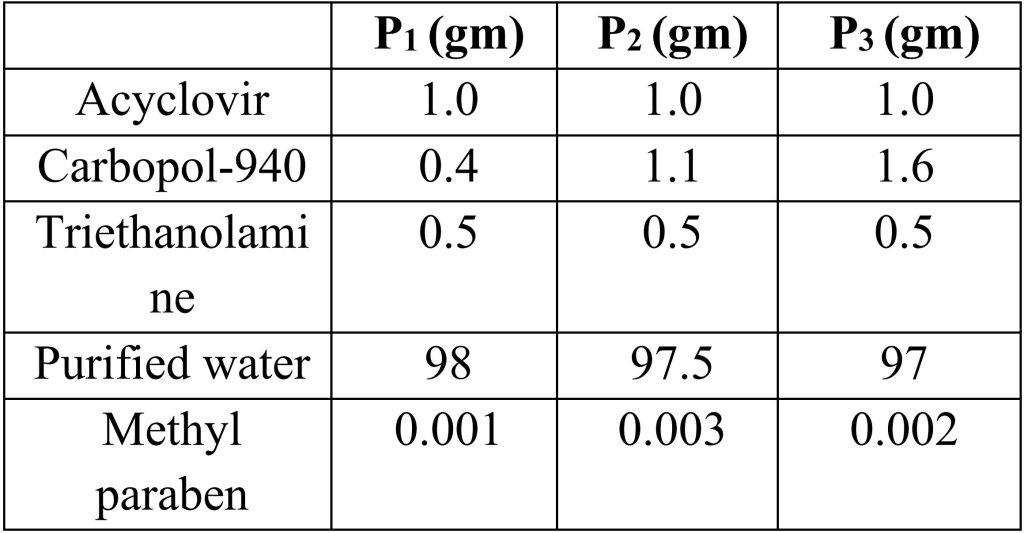
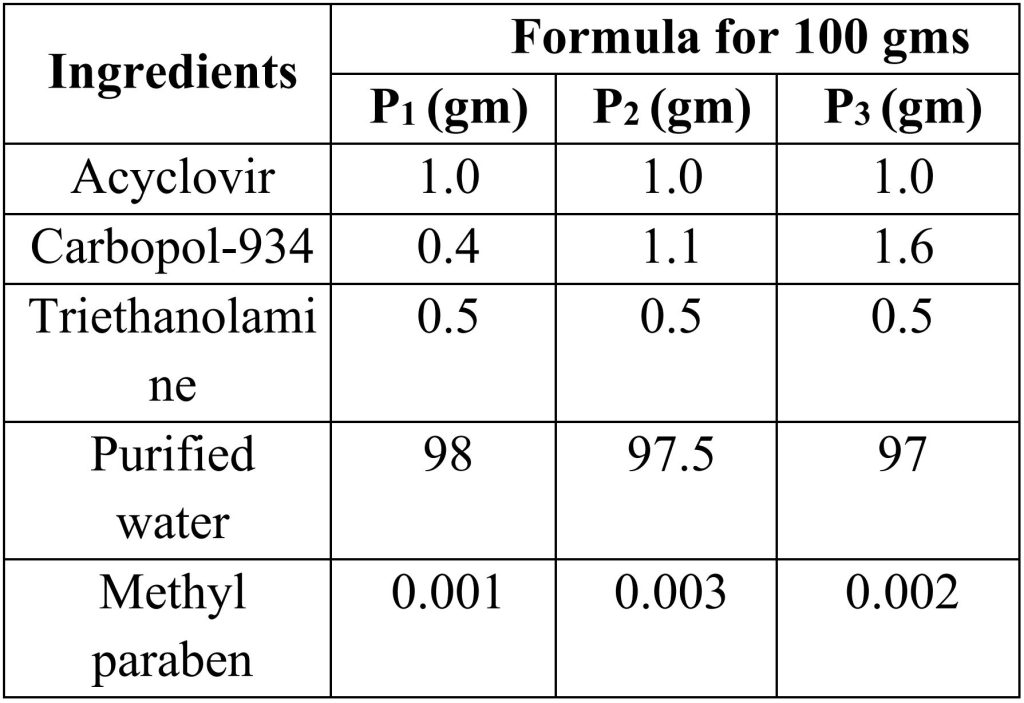
Acyclovir gels are prepared by using different polymers like Carbopol 934, carbopol 940, Hydroxy propyl methyl cellulose, etc. different concentration of polymer used in preparation of gels.
2.2 Preparation of Carbopol-934 gels:
Procedure:
a) Accurately weigh the quantity of acyclovir is mixed in purified water with constant stirring and heat it at 500C.
b) Add methyl paraben as preservative.
c) Add carbapol-934 to solution with continuous stirring at 500C temperature.
d) Then add triethanolamine to the solution to maintain pH, stir until a clear gel was obtained.
Download the full article as PDF here Preparation and Characterization of an Antiviral Gel
INTERNATIONAL JOURNAL OF PHARMA PROFESSIONAL’S RESEARCH, Preparation and Characterization of an Antiviral Gel, Shivam Tayal, Pradyumn Tiwari, Vinod Sahu, Shivang Sharma, School of Pharmacy, ITM University, Gwalior, India, IJPPR (2023), Vol. 14, Issue 4,Volume 14, Issue 4, October 2023
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free: