Press-Coated Aceclofenac Tablets for Pulsatile Drug Delivery: Formulation and In Vitro Evaluations
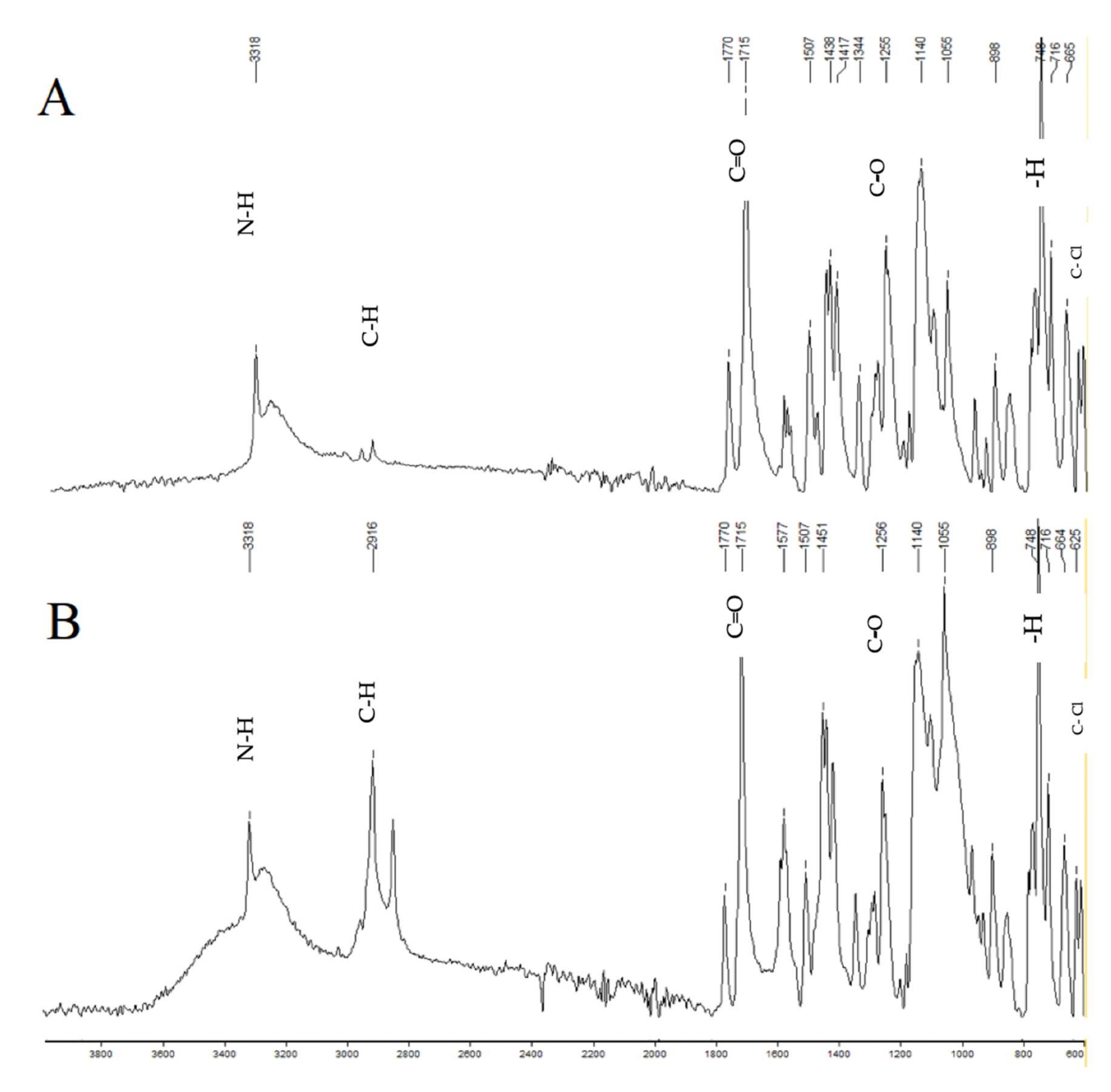
The symptoms of some diseases show circadian rhythms, such as the morning stiffness associated with pain at the time of awakening in rheumatoid arthritis. Therapy for such diseases doesn’t require immediate release or sustained release of medicament. In such therapies, pulsatile drug release is more suitable with a programmed drug release. The purpose of this research was to formulate press-coated aceclofenac tablets for pulsatile drug delivery with a distinct delay time of no drug release and release of the drug when it is more likely desired (i.e., after 5 to 6 h). Immediate release core tablets having aceclofenac were formulated.
Three formulations, F1, F2, and F3, were prepared with variable concentrations of sodium croscarmellose. Pre- and post-compression tests were performed on the core tablets. The selection criteria included the lowest disintegration time as a requirement of pulsatile drug delivery with an immediate release core and a delayed release coat. The disintegration times of F1, F2, and F3 were 120 s, 60 s, and 15 s, respectively. Therefore, the F3 formulation was selected as the core tablet formulation because it had the shortest disintegration time (15 s). The core tablets were press-coated using different polymers, such as HPMC K100M, Eudragit L100, HEC, and HPMC E5.
The polymers were used in the coatings to hinder the release of the core for the desired time. 36 formulations of polymer were prepared: A1 to A10 had HPMC K100M and Avicel PH102; formulations B1 to B6 had HPMC K100M, Eudragit L100, and Avicel PH102; formulations C1 to C7 had HPMC K100M and hydroxyethyl cellulose; formulations D1 to D7 had HPMC K100M and HPMC E5; and formulations E1 to E6 had changed the coating weight of the formulation used for D6 (having HPMC K100M and HPMC E5 in the ratio of 12.5% to 87.5%). Evaluations of the press-coated tablets were carried out through thickness, hardness, weight variation, friability, and in vitro dissolution tests. These parameters concluded that the formulation of E6, having HPMC K100M and HPMC E5 in the ratio of 12.5% to 87.5% at 600 mg weight, was the most optimum formulation as it showed 3.5% drug release after 4 h, 21.4% drug release after 5 h, and 99.27% drug release after 6 h.
Continue reading here
About this article: Rashid, R.; Zaman, M.; Ahmad, M.; Khan, M.A.; Butt, M.H.; Salawi, A.; Almoshari, Y.; Alshamrani, M.; Sarfraz, R.M. Press-Coated Aceclofenac Tablets for Pulsatile Drug Delivery: Formulation and In Vitro Evaluations. Pharmaceuticals 2022, 15, 326. https://doi.org/10.3390/ph15030326
Materials
Aceclofenac was obtained from Highnoon Laboratories Ltd. (Lahore, Pakistan); Avicel PH102 was obtained from JRS Pharma (Rosenberg, Germany); croscarmellose sodium was obtained from Mingtai (Taoyuan City, Taiwan ); magnesium stearate was obtained from Peter Greven Asia (Selangor, Malaysia); sunset yellow lake E110 was obtained from Roha, A JJT Group Company (Nagpur, India); HPMC K100M and HPMC E5 were obtained from Zhongbao Chemicals Co., Ltd (Hangzhou, China); Eudragit L100 was obtained from Evonik (Essen, Germany); and hydroxyethyl cellulose was obtained from Sigma Aldrich (St. Louis, MO, USA). All excipients were of standard pharmacopoeia grade and all chemical reagents used for analysis were of analytical grade. HPMC is available in a variety of viscosity grades. Viscosities of the aqueous solution of methocel are measured at 20 °C. The viscosity of HPMC K100M is 100 cp, while the viscosity of HPMC E5 is 5 cp. Hydroxyethyl cellulose is soluble in water, is a non-ionic polymer, and, in a 2% solution, has a viscosity of 80–125 cp at 20 °C. Eudragit L100 is a delayed release polymer that dissolves at 6.0 pH, and croscarmellose sodium (Ac-di-sol) is a cross-linking polymer derived from carboxymethylcellulose sodium. Ac-di-sol is used as a disintegrant. Microcrystalline cellulose is partially depolarized, purified cellulose that is available as a white, tasteless, odorless, crystalline powder having porous particles. Microcrystalline cellulose is available in different particle sizes and moisture grades having different applications and properties. Avicel PH102 has a particle size of 100 µm. HPMC K100M, HPMC E5, and HEC were selected for the coatings because they have the tendency to swell, form gel, and erode when they come in contact with water. With such properties, they play a role in delaying the release of the inner core. Eudragit L100 is a pH-dependent polymer and only resists drug release in the acidic media of the stomach.

