Neusilin Redefining Silica´s Potential in Solid Oral Dosage Forms

Neusilin
WHAT IS IT?
Neusilin® UFL2 is an ultra fine powder of magnesium aluminometasilicate and is widely accepted as a problem solving excipient for oral solid dosage forms.

Make harder tablets and protect it from humidity by adding 0.5-5% Neusilin® UFL2 to a lactose or mannitol formulation.
In this newsletter, we introduce you to a new study where 2% Neusilin® UFL2 is used as excipient to improve hardness of acetaminophen tablets and to protect the tablets from deterioration by moisture. Tablet hardness does not decrease significantly even under humid conditions.
WHY IS IT BENEFICIAL?
Improve Tablet Strength
 Adding 2% Neusilin® UFL2 to tablet formulations can enhance tablet hardness and maintain it even under humid conditions.
Adding 2% Neusilin® UFL2 to tablet formulations can enhance tablet hardness and maintain it even under humid conditions.
Satisfactory Dissolution Rates
 Tablets with Neusilin® UFL2 exhibited satisfactory dissolution rates, ensuring effective drug release.
Tablets with Neusilin® UFL2 exhibited satisfactory dissolution rates, ensuring effective drug release.
Protection from Moisture
 Neusilin® UFL2 acts as a protective agent, preventing tablet deterioration caused by moisture.
Neusilin® UFL2 acts as a protective agent, preventing tablet deterioration caused by moisture.
Improved Stability
 Formulations using Neusilin® UFL2 with different excipients showed better hardness retention compared to formulations without Neusilin® UFL2.
Formulations using Neusilin® UFL2 with different excipients showed better hardness retention compared to formulations without Neusilin® UFL2.
Reduced tablet weight variation
 Addition of Neusilin® UFL2 reduced the coefficient of variation (CV) of tablet weight.
Addition of Neusilin® UFL2 reduced the coefficient of variation (CV) of tablet weight.
Excellent Flow Aid
 Neusilin® UFL2 helps improve flow even at concentrations as low as 0.5%.
Neusilin® UFL2 helps improve flow even at concentrations as low as 0.5%.
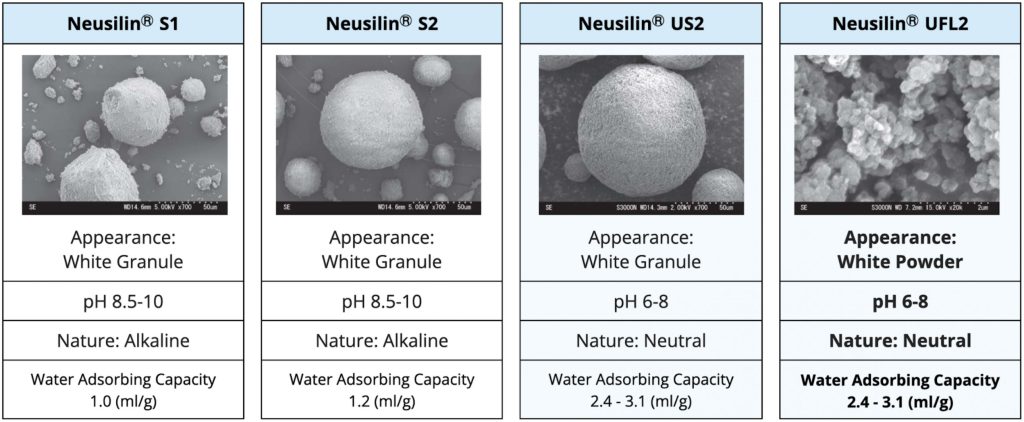
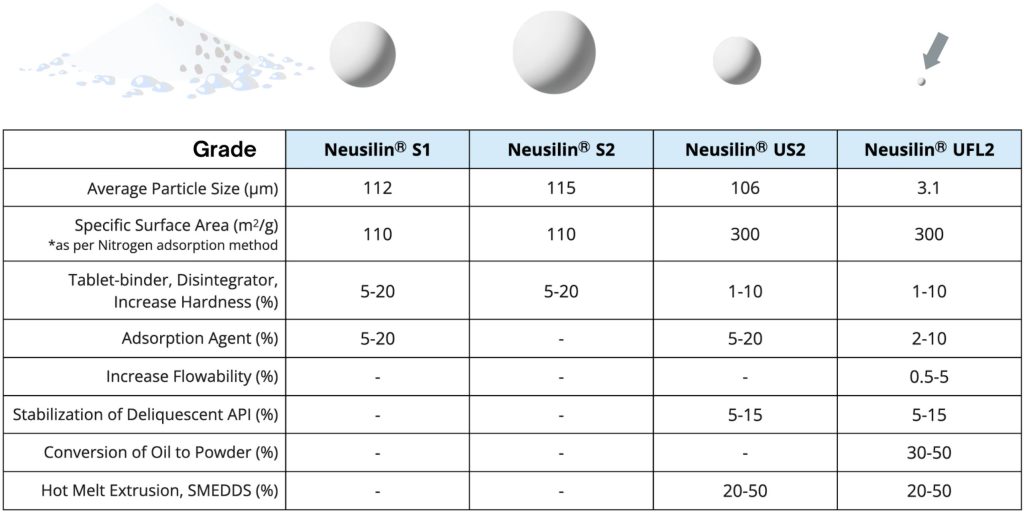
CHARACTERISTICS AND SPECIFICATIONS
The US2 and UFL2 grades of Neusilin® have a large specific surface area, allowing them to adsorb oils up to three times their own weight. This property makes them effective in tablet production. These grades also provide better flowability and result in harder tablets at low compression force. Unlike traditional Magnesium Aluminum Silicates, which have an alkaline pH, US2 and UFL2 have a neutral pH, making them compatible with a broader range of active pharmaceutical ingredients (APIs).

INVESTIGATING THE IMPACT OF NEUSILIN® ON TABLET
STABILITY
Acetaminophen tablets of varying compositions with and without Neusilin® UFL2 were prepared by DC process and evaluated for hardness and dissolution at different conditions of stability.
Process:
The batch size of formulation was set at 1000 tablets(150 g). The materials were placed in a polyethylene bag and mixed thoroughly. Tablets were produced by single punch tabletting equipment (N-30EX model, Okada Seiko Co., Ltd.; tablet diameter: 7.0 mm, compression force: 700 kgf). Tablet properties including weight, thickness and hardness were measured using 10, 5, and 5 tablets respectively. The dissolution test was carried out by the paddle method (50 rpm), using 3 tablets in 900 ml of water. The dissolution rate was measured spectrophotometrically at wavelength of 244 nm.
Results:
Addition of 2% Neusilin® UFL2 increased the tablet hardness irrespective of the filler-binder excipients used formulations #2, #3 and #5). Furthermore, with Super-tab as excipient, Neusilin® UFL2 reduced the coefficient of variation (CV) of tabletweight from 1.34% to 0.78% and 0.9% (formulations #1-#3). The dissolution rate met a criterion of 85% or more in 15 minutes in all formulations.
Results:
Accelerated stability tests and stability test at room temperature were performed for a period of one month with 30 tablets in high-density polyethylene (HDPE) bottles (sealed or open). The hardness and dissolution profiles of tablets are shown in the following Table.
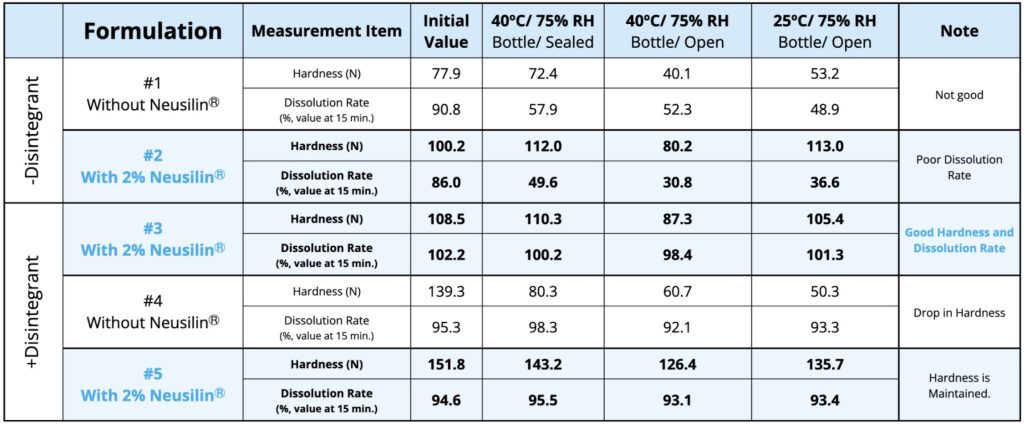
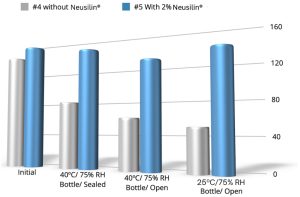 In formulations #1 and #4 in which Neusilin® UFL2 was not added, the hardness decreased significantly under accelerated stability test (bottle/open, bottle/sealed) and in stability test at room temperature (bottle/open) conditions. The formulations without Neusilin® showed a stark drop in hardness as opposed to those containing Neusilin® UFL2. It was noteworthy to look at formulation #5 where Neusilin® UFL2 was added with Parteck M200 as excipient and the hardness remained above 100 N at all stability test conditions.
In formulations #1 and #4 in which Neusilin® UFL2 was not added, the hardness decreased significantly under accelerated stability test (bottle/open, bottle/sealed) and in stability test at room temperature (bottle/open) conditions. The formulations without Neusilin® showed a stark drop in hardness as opposed to those containing Neusilin® UFL2. It was noteworthy to look at formulation #5 where Neusilin® UFL2 was added with Parteck M200 as excipient and the hardness remained above 100 N at all stability test conditions.
Continue reading and see the full Pharmaceutical Technical Newsletter on “Special Issue – Neusilin Redefining Silica´s Potential in Solid Oral Dosage Forms” here:
(click the picture to download the technical newsletter)
Source: Fuji Chemical Industries technical newsletters “Special Issue – Neusilin Redefining Silica´s Potential in Solid Oral Dosage Forms”
Read also the other Technical Newsletter of Fuji Chemical Industries here:
- Issue 04 – Vitamin E Tablets with Fujicalin®
- Issue 05 – F-Melt® oral disintegrating tablets Simvastatin
- Issue 06 – The Cost-Effective Solution For Producing High-Quality Orally Disintegrating Tablets (ODTs)
- Issue 07 – Unveiling Neusilin US2´s Prowness
- Special Issue – Mitigating Nitrosamine Risks in Drug Products
Do you need more information or a sample of Fuji Chemical Industries excipients?