Single and Multitarget Systems for Drug Delivery and Detection: Up-to-Date Strategies for Brain Disorders
Abstract
This review summarizes the recent findings on the development of different types of single and multitarget nanoparticles for disease detection and drug delivery to the brain, focusing on promising active principles encapsulated and nanoparticle surface modification and functionalization. Functionalized nanoparticles have emerged as promising tools for the diagnosis and treatment of brain disorders, offering a novel approach to addressing complex neurological challenges. They can act as drug delivery vehicles, transporting one or multiple therapeutic agents across the blood–brain barrier and precisely releasing them at the site of action. In diagnostics, functionalized nanoparticles can serve as highly sensitive contrast agents for imaging techniques such as magnetic resonance imaging and computed tomography scans. By attaching targeting ligands to the nanoparticles, they can selectively accumulate in the affected areas of the brain, enhancing the accuracy of disease detection. This enables early diagnosis and monitoring of conditions like Alzheimer’s or Parkinson’s diseases. While the field is still evolving, functionalized nanoparticles represent a promising path for advancing our ability to diagnose and treat brain disorders with greater precision, reduced invasiveness, and improved therapeutic outcomes.
Introduction
The prevalence of neurodegenerative and neuropsychiatric disorders is increasing dramatically. According to the World Health Organization (WHO), in 2023, more than 55 million people have dementia worldwide, with Alzheimer’s disease (AD) being the most common form (60 to 70% of cases) [1], while the data from 2019 indicate that the global prevalence of Parkinson’s disease (PD) was 8.5 million individuals [2]. The pathogenesis of AD is a complex process that remains unclear. The aggregation of beta-amyloid (Aβ) to form senile plaques and oligomers of Aβ, aggregation of tau to form neurofibrillary tangles (NFTs), change in acetylcholine levels, oxidative stress, and neuroinflammation are the main hallmarks in AD. However, AD is still managed with symptomatic treatments consisting of acetylcholinesterase inhibitors (AChEIs) (donepezil, rivastigmine, and galantamine) and/or N-methyl-D-aspartate (NMDA) receptor antagonists (memantine). Although being a topic of debate, Aducanumab, an Aβ-directed monoclonal antibody, was granted accelerated approval by the US Food and Drug Administration (FDA) in 2021 as the first disease-modifying therapy for AD. Other mechanisms and pathways of interest are being explored to develop new therapies, including focusing in other neurotransmitters, metabolism, the neuroimmune system, the microbiome, and the activity of a variety of nutraceuticals [3]. PD is characterized by the presence of Lewy bodies (LBs) and Lewy neurites (LNs), which are intraneuronal inclusion bodies observed in postmortem histopathological examinations of the brains of PD patients. The prodromal phase of PD is marked by a variety of non-motor symptoms that can precede the emergence of classical motor symptoms by several years. These early symptoms encompass issues such as constipation, gastric motility problems, sleep disruptions, depression, anxiety disorder, and cognitive impairments. As the disease progresses to stages 3 and 4, it affects key brain regions like the substantia nigra, thalamus, and amygdala, and the characteristic motor symptoms become clinically apparent. At stages 5 and 6, the pathology extends to sensory and motor areas in the neocortex, culminating in the most pronounced manifestation of the disease. In advanced stages of PD, patients may experience more severe motor symptoms, including instability, an increased risk of falls, difficulty swallowing (dysphagia), and non-motor symptoms like dementia and psychosis [3]. Currently, dopaminergic drugs are used to control the progression of the disease [4].
Exploring novel neuroprotective drugs with different mechanisms of action from those already used, combining drugs aiming at a multitarget approach, or finding new delivery routes to deliver the currently available drugs more efficiently to the brain are three possible ways to meet the requirements of the patients. In spite of new or combined mechanisms of action, multitarget therapy can offer multiple advantages, being more efficient than the conventional single-target treatment approaches. This can be achieved either using “cocktail drug–multiple targets” or “one compound–multiple targets” approaches [5]. For instance, combining α-tocopherol (an antioxidant) with donepezil (a cholinesterase inhibitor) showed promising results in an animal model of AD [6], as well as the co-delivery of BACE1 antisense sh-RNA and D-peptide, which induced both a reduction in Aβ plaque deposition and the inhibition of p-tau-related fibril formation in double transgenic (Tg) mice ((APPswe, PSEN1dE9)85Dbo/Mmjax), respectively [7]. Moreover, it has been shown that neuronal survival and repair processes were promoted by the combinatory effect of curcumin and docosahexaenoic acid [8]. Glinz et al. conducted a systematic review and meta-analysis of nine randomized controlled trials (2604 patients) comparing the clinical efficacy and safety of a combination therapy of AChEIsand memantine to monotherapy with either substance in patients with moderate to severe AD. The authors concluded that combined therapy had a significantly greater effect on cognition than AChEI monotherapy [9]. The benefits of combined therapy for AD were also highlighted by Kabir et al. [10]. Hybrid compounds have been designed for AD treatment by incorporating multiple known pharmacophores. For instance, compounds with dual-acting effects such as AChEI and butyrylcholinesterase inhibitor (BuChEI); AChEI and β-secretase (BACE 1) inhibitor; AChEI and glycogen synthase kinase 3 beta (GSK-3β) inhibitor; AChEI and NMDA antagonist; AChEI and serotonin receptor (5-HT4R) partial agonist; AChEI and serotonin transporter (SERT) inhibitor; ChEI and antioxidant; and AChEI and MAO-B inhibitor, among others, have been developed [5,11]. NLX-112, a non-dopaminergic drug (a highly selective 5-HT1A receptor agonist), is a dual-effect drug, acting both on dyskinesia and the movement symptoms of PD [12].
Concerning neuropsychiatric disorders, depression and anxiety are the most common neuropsychiatric disorders, affecting approximately 280 [13] and 301 million people [14], respectively. Progress in discovering and developing new and improved psychiatric drugs has been slow and disappointing. The vast majority of currently prescribed drugs are arguably no more effective than the first generation of psychiatric drugs introduced in the 1950s and 1960s [15]. The etiology of depression (major depressive disorder) and anxiety are still not clear, but it is generally believed that they are multifactorial diseases caused by the interaction of social, psychological, and biological aspects. The symptoms include avoiding contact with friends; neglecting hobbies and interests; having difficulties in home, work, or family life; continuous low mood or sadness; feeling hopeless and helpless; having low self-esteem; feeling irritable and intolerant of others; having no motivation or interest in things; finding it hard to make decisions; moving or speaking more slowly than usual; changes in appetite or weight; unexplained aches and pains; and disturbed sleep. Despite several monoaminergic drugs being available on the market, 1/3 of depressive patients are unresponsive to this kind of treatment, Spravato® (S-ketamine, an NMDA receptor antagonist) being their only hope [16,17]. However, concerns such as the transient adverse effects and potential misuse liability of Spravato® (Janssen: Pharmaceuticals, Inc., Titusville, NJ, USA) have limited its widespread use [18]. Henssler et al. [19] performed a meta-analysis of 39 trials comprising 6751 patients and found that combined antidepressant therapy using a reuptake inhibitor with an antagonist of presynaptic α2-autoreceptors (mianserin, mirtazapine, trazodone) was associated with significantly superior treatment outcomes compared with antidepressant monotherapy.
Symptoms of anxiety include feeling restless; being easily fatigued or irritable; having difficulty in concentrating; having unexplained pains (e.g., headaches, muscle aches, stomachaches); having difficulty in controlling feelings of worry; and having sleep problems. The first-line treatment for anxiety is based on selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), and benzodiazepines that target the gamma-amino butyric acid (GABA) receptor. However, the drugs currently in clinical development for the treatment of anxiety disorder include other mechanisms of action, namely NMDA receptor antagonism, negative allosteric modulation of metabotropic glutamate receptors 1–5, sodium channel inhibition, vasopressin receptor 1A antagonism/agonism, antagonism of orexin receptors 1,2, and agonism of neuropeptide Y receptors 1,2,5 [20,21].
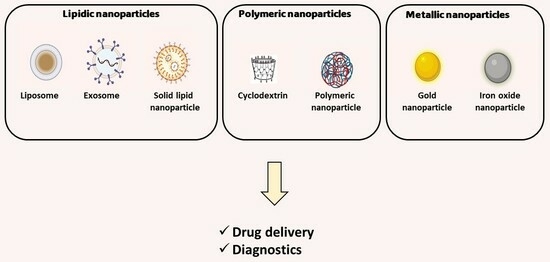
Other important aspect deals with the delivery route of drugs to the brain. Developing an efficient therapeutic system for brain disorders faces a significant challenge due to the presence of the blood–brain barrier (BBB), a barrier that separates the blood and the brain interstitial fluid and obstructs the passage of drugs, peptides, and large molecules into the brain [22]. Several strategies have been developed to increase the passage of the drugs through the BBB. Thus, this review paper provides a comprehensive analysis of the latest developments in nanoparticle-based drug delivery and diagnostics for brain disorders, encompassing neurodegenerative (AD and PD) and neuropsychiatric conditions (depression and anxiety). An array of nanoparticles, including lipid nanoparticles (liposomes, exosomes, solid lipid nanoparticles, and nanoemulsions), polymeric nanoparticles (PLGA, chitosan, alginate-based polymeric nanoparticles, and cyclodextrins), as well as magnetic nanoparticles, have emerged as promising tools in the quest to enhance the therapeutic and diagnostic outcomes for these challenging diseases. Strategies to functionalize these nanoparticles will also be discussed in the next sections (Figure 1).
Download the full article as PDF here Single and Multitarget Systems for Drug Delivery and Detection
or read it here
Grosso, C.; Silva, A.; Delerue-Matos, C.; Barroso, M.F. Single and Multitarget Systems for Drug Delivery and Detection: Up-to-Date Strategies for Brain Disorders. Pharmaceuticals 2023, 16, 1721. https://doi.org/10.3390/ph16121721
Read more on “Alginates as Pharmaceutical Excipients” here:


