Formulation and in vitro skin diffusion of colchicine using different drug delivery vehicles
Introduction
Historically known as “the disease of kings,” gout is one of the oldest known types of arthritis, affecting more than 40 million people worldwide, prevalent in patients between 30 and 50 years of age, and frequently experienced more by males than females [[1], [2], [3], [4]]. It is a result of monosodium urate crystal deposition in tissues (cartilage and joints), consequently causing an immune response [[5], [6], [7]]. Clinical manifestations include severe pain and inflammatory attacks affecting common joints, chronic joint damage and tophaceous deposits of monosodium urate crystals in the skin and joints [1,4]. Gout is an understood and manageable rheumatic disease, the treatment of which is mostly non-steroidal anti-inflammatory drugs (NSAIDs, i.e., indomethacin and naproxen) due to their efficacy and low toxicity when administered orally [7,8]. Furthermore, there are three different active pharmaceutical ingredients (APIs) used for the treatment of gout, each having different mechanisms of action, which includes allopurinol: an API that inhibits uric acid production, probenecid: an API that increases uric acid excretion and finally, colchicine: an API, which does not affect uric acid metabolism or elimination [9].
Colchicine is an API used in the treatment of acute gouty arthritis as well as prophylaxis for patients who experience reoccurring gout attacks [8]. Colchicine is an alkaloid that is isolated from Colchicine autumnale (autumn crocus), a plant that possesses anti-inflammatory properties [6]. When administered orally, colchicine, presents a decreased benefit-toxicity ratio, as it results in dose-dependent gastrointestinal adverse effects, such as nausea, vomiting, diarrhoea and abdominal pain in 50–80% of patients before achieving an outcome of gout relief [6,8]. Colchicine has a narrow therapeutic toxicity window and can be very toxic when used inappropriately, where gastrointestinal symptoms are usually the first feature of colchicine toxicity [7]. Due to colchicine’s small therapeutic window, the transdermal delivery of colchicine may subsequently improve patient compliance through the relief of gout symptoms, while still avoiding any dose-dependent gastrointestinal adverse effects. This study will focus on the transdermal delivery of colchicine, which subsequently may improve patient compliance to relieve gout symptoms by avoiding any dose-dependent gastro-intestinal adverse effects.
Download the full article as PDF here Formulation and in vitro skin diffusion of colchicine using different drug delivery vehicles
or read it here
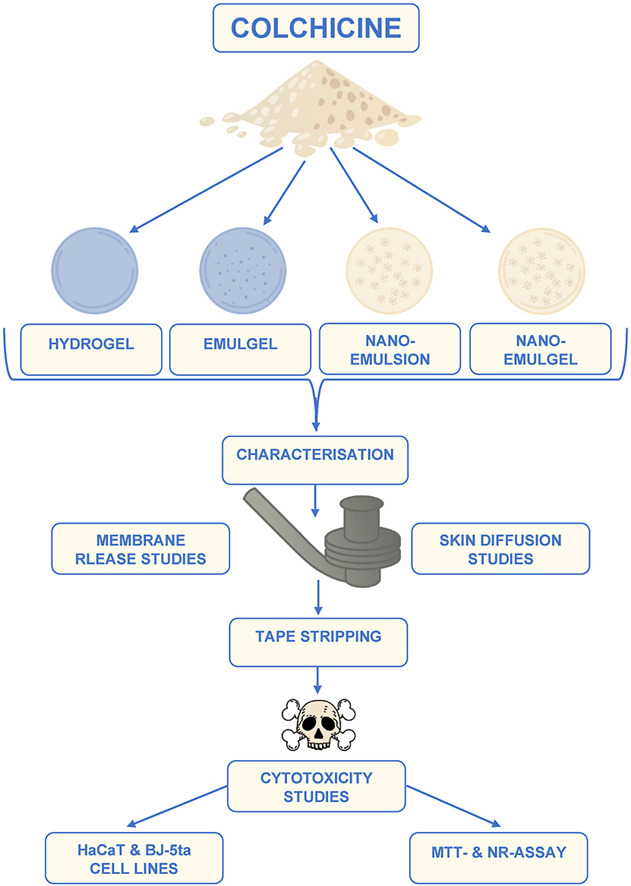
Materials
Colchicine came from DB Fine Chemicals (Sandton, South Africa). All formulated hydrogels, emulgels and nano-emulgels contained Carbopol® Ultrez 20, purchased from Lubrizol (Durban, South Africa). Each emulgel, nano-emulsion and nano-emulgel contained surfactants, Span® 60 (lipophilic surfactant) and Tween® 80 (hydrophilic surfactant) (both obtained from Sigma-Aldrich, Johannesburg, South Africa) and evening primrose oil (EPO) (Scatter Oils, Johannesburg, South Africa). The phosphate buffer solution (PBS; pH 7.4) was prepared with sodium hydroxide (NaOH) and potassium dihydrogen phosphate (KH2PO4), both purchased from Sigma-Aldrich (Johannesburg, South Africa). Throughout this study, ultrapure (UP) water was used and obtained from the Direct Pure® Ultrapure laboratory water purification system (Merck-Millipore, Midrand, South Africa). During each high-performance liquid chromatography (HPLC) experiment, there was chromatography grade acetonitrile and methanol used in addition to analytical grade formic acid, all purchased from Associated Chemical Enterprises (ACE) (Johannesburg, South Africa). Dow Corning® high vacuum grease, Whatman® filter paper and Parafilm® (obtained from Separations, Randburg, South Africa) were used during membrane release and skin diffusion studies. Dulbecco’s Modified Eagle’s Medium (DMEM) (purchased from HyClone, Separations, Johannesburg, South Africa), 1% non-essential amino acids (NEAAs), 1% penicillin/streptomycin (10000 U/ml) (both obtained from Lonza, Whitehead Scientific (Pty) Ltd, Cape Town, South Africa), 10% foetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Johannesburg, South Africa), hygromycin (Sigma-Aldrich), Trypsin-Versene® ethylenediaminetetraacetic acid (EDTA) (Lonza, Whitehead Scientific (Pty) Ltd, Cape Town, South Africa) and Trypan Blue solution (0.4%) (HyClone, Separations, Johannesburg, South Africa) were utilised during the cytotoxicity studies.
Micaela Ponte, Wilna Liebenberg, Minja Gerber, Formulation and in vitro skin diffusion of colchicine using different drug delivery vehicles, Journal of Drug Delivery Science and Technology, Volume 88, 2023, 104898, ISSN 1773-2247,
https://doi.org/10.1016/j.jddst.2023.104898.

