The Use of Sodium Alginate in Extended Release Tablet Formulations

Extended release tablets are commonly based on partially synthetic cellulose ethers, such as hypromellose (HPMC), hydroxyethyl cellulose (HEC), or hydroxypropyl cellulose (HPC). Alginates offer an interesting, all-natural alternative to these polymers. The aim of the present study was to investigate the effect of particle size, concentration, and viscosity of various grades of sodium alginate on the performance of modified release tablets. Caffeine tablets with extended release over 8-10 hours were successfully prepared by direct compression with VIVAPHARM® sodium alginate.
Introduction
Therapeutic requirements as well as patient convenience make it desirable to present dosage forms with prolonged release of the active principal. Among the available formulation techniques, the so-called hydrophilic matrix is the easiest and most cost-efficient form. The key of hydrophilic matrices are the active components ingredient(s) and a sufficient quantity (usually 20-30 %) of a hydrophilic polymer. Upon contact with water, the polymer will form a gel layer at the interface of the release medium and the tablet core. Drug release will then occur controlled by diffusion through the gel layer and/or by erosion of the same.
A number of excipient properties are critical to the performance of extended release systems:
The viscority of the polymer affects the strength of the release-controlling gel layer. It has, therefore, a significant impact on the release rate. The concentration of the extended release polymer in a hydrophilic matrix has to exceed a certain level, in order to enable the reproducible formation of a robust gel structure [1]. The particle size distribution (PSD) of the release-controlling polymer will have an influence on the velocity of the gel formation. I.e. in case of coarse particles, gel-formation will take longer the than for a material of finer PSD. The more time elapses before a consistent gel is formed, the more API can diffuse out of the tablet in an uncontrolled manner. This phenomenon is often referred to as the “burst effect”.
Aim of the Study
As shown in the introduction, viscosity, concentration, and particle size have an impact on the drug release kinetics. The goal of this study was to understand the interactions of these parameters in order to develop criteria for the selection of alginate sodium grades for extended release tablets made by direct compression. In addition, suitable formulations were compared against corresponding formulations based on HPMC, the most widely which is used release-controlling polymer in hydrophilic matrix tablets
Materials and Methods
Modified release caffeine tablets were produced by direct compression using caffeine as a model API, different levels of VIVAPHARM® sodium alginate or HPMC as release-controlling polymer, silicified microcrystalline cellulose (SMCC) as a filler-binder and magnesium stearate as the lubricant. For comparison, immediate release tablets were produced by direct compression of caffeine with the all-in-one excipient PROSOLV EASYtab Nutra CM ® comprising filler-binder, flow aid, disintegrant, and lubricant.
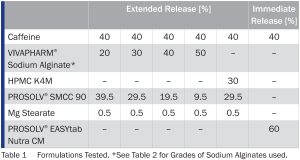
Results Discussion
The Effect of Viscosity
The viscosity of release-controlling polymers correlates with their polymer chain length and consequently with the robustness of the gels they form at higher concentrations. The effect of viscosity is shown in Figure 1 for three grades of sodium alginate having the same particle size but spanning a range of 100 – 750 mPa*s in terms of viscosity. All formulations contained 20 % alginate.
See and download the full technical information as PDF here:

Source: JRS Pharma Whitepaper “The Use of Sodium Alginate in Extended Release Tablet Formulations”

