An innovative carrier for the formulation of amorphous solid dispersion by hot-melt extrusion with no further downstream processes: a case study with indomethacin

The aim of this work was to study the possibility to use SepitrapTM as a carrier for the formulation of amorphous solid dispersions by HME (hot melt extrusion) processing aiming solubility enhancement of poorly water–soluble drugs. SepitrapTM is a microencapsulated powder solubilizer designed to simplify the manufacture of drugs in oral solid forms, not yet tested for this purpose. The performance of SepitrapTM was evaluated in HME processing for amorphous solid dispersions of poorly-water soluble drugs with indomethacin as a model drug.
The study was conducted using a twin-screw extruder, two compositions of SepitrapTM and different loads of indomethacin, demonstrating that SepitrapTM could represent a new range of carriers for amorphous solid dispersions for HME processing, reducing necessary downstream steps such as grinding.
Read more here
(2024) An innovative carrier for the formulation of amorphous solid dispersion by hot-melt extrusion with no further downstream processes: a case study with indomethacin., Pharmaceutical Development and Technology, DOI: 10.1080/10837450.2024.2306802
Read more articles on Indomethacin here:
- Wet granulation of co-amorphous indomethacin systems
- Numerical simulation of five different screw configurations used during the preparation of hot-melt extruded Kollidon® and Soluplus® based amorphous solid dispersions containing indomethacin
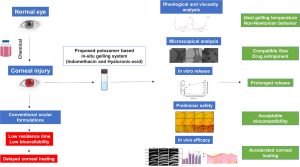
- Thermoresponsive in-situ gel containing hyaluronic acid and indomethacin for the treatment of corneal chemical burn