Enhanced stability and skin permeation of ibuprofen-loaded solid lipid nanoparticles based binary solid lipid matrix: Effect of surfactant and lipid compositions
Hypothesis
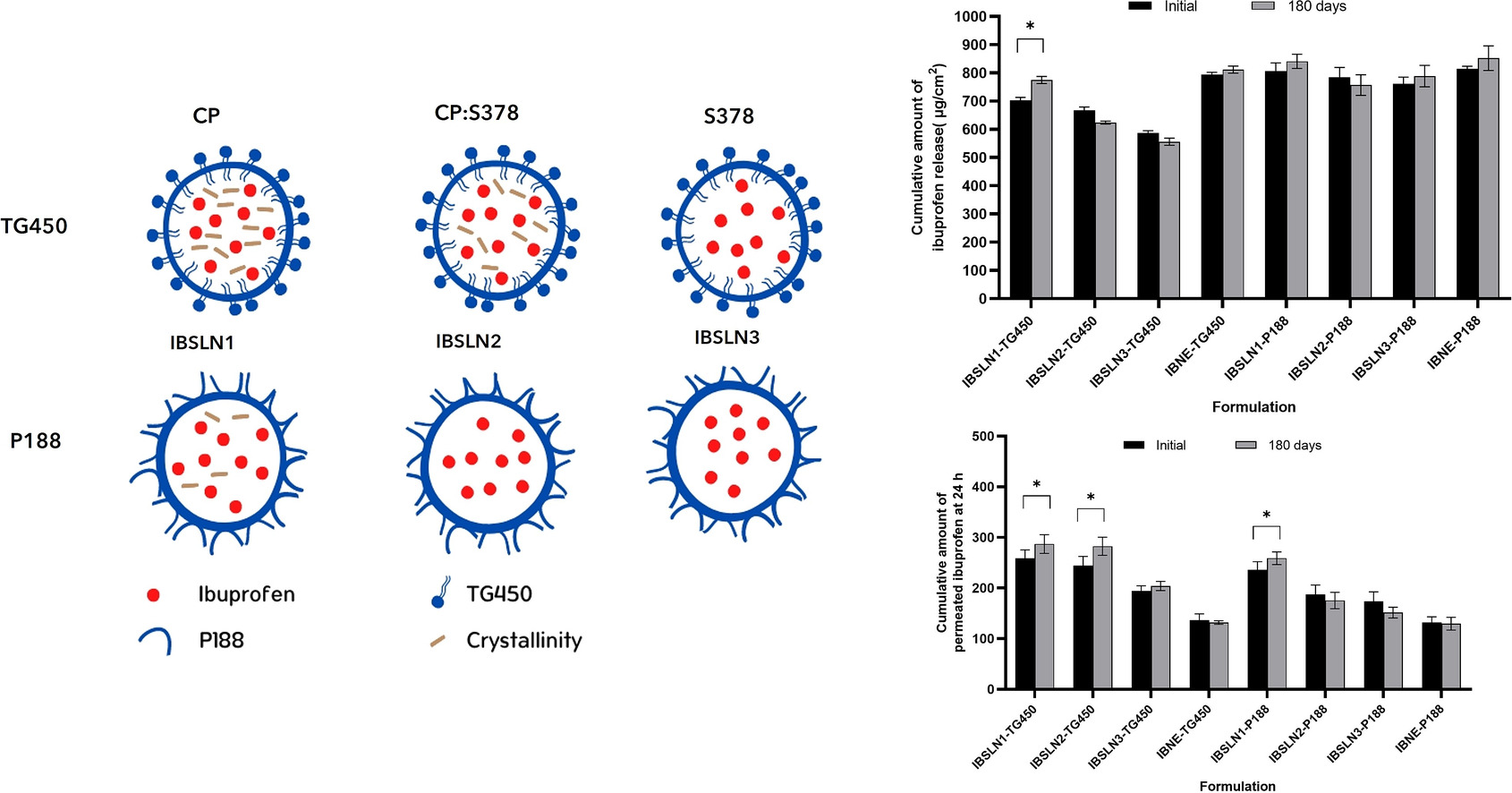
The type of emulsifier selected has an impact on the physicochemical properties of solid lipid nanoparticles (SLNs). This study was designed to compare the effects of emulsifiers on the physicochemical properties and in vitro skin performance of SLNs prepared from a binary mixture of Softisan® 378 (S378) and cetyl palmitate (CP) to those of SLNs prepared from only CP and S378.
Highlights
- The emulsifier types affect the properties of ibuprofen-loaded solid lipid nanoparticles in a binary solid lipid matrix.
- Using an appropriate emulsifier and/or adding S378 in the CP matrix ensures stability of ibuprofen-loaded nanoparticles.
- The supercooled melts exhibited a greater occlusion factor than nanoemulsions.
- Lipid nanoparticle stability under various storage conditions relies on the composition and crystallinity of the lipid matrix.
Experiments
SLNs were prepared from CP, S378, or a binary mixture of CP and S378 (1:1 w/w) as the lipid phase and stabilized with Tego®Care 450 (TG450) or poloxamer 188 (P188) containing 1.0% w/w ibuprofen loading. The physicochemical properties including the particle size, polydispersity index (PDI), zeta potential (ZP), encapsulation efficiency (E.E.), crystallinity (%CI), and polymorphism were determined after production and after storage for 180 days under different conditions. In addition, in vitro drug release and permeation through human skin was studied after production and storage at room temperature for 180 days.
Finding
The particle sizes of ibuprofen-loaded SLNs (IBSLNs) stabilized with P188 (IBSLN-P188) were smaller than those of SLNs stabilized with TG450 (IBSLN-TG450) (p < 0.05). After 180 days, the particle sizes of the IBSLNs were slightly increased compared to those at the initial time but were <250 nm. The IBSLN-TG450 sample showed a higher %CI than IBSLN-P188 prepared with similar propotions of CP and S378, and ibuprofen crystals were observed in the IBSLN1-TG450 sample after storage at 4 °C for 180 days. Based on the result of the in vitro release study and the in vitro skin permeation test, the addition of S378 into the CP-matrix modified ibuprofen release and skin permeation both permeated ibuprofen through the epidermis and retained ibuprofen in the epidermis. In addition, the storage time affected the release and skin permeation of ibuprofen from the SLNs, which depended on the composition of the IBSLNs.
2.1. Materials
CP was obtained from SABO S.p.A. (Levate (BG), Italy). Softisan® 378 (S378, caprylic/capric/myristic/stearic triglycerides) and Miglyol 812 (M812, caprylic/capric triglycerides) were purchased from Sasol GmbH (Hamburg, Germany). Tego®Care 450 (TG450, polyglyceryl-3 methylglucose distearate) was gifted from Evonik Industries AG (Essen, Germany). Poloxamer 188 (P188) was gifted from BASF (Ludwigshafen, Germany). Ibuprofen was purchased from HuBei Granules-Biocause Pharmaceutical Co., Ltd. (Hubei, China). Sodium chloride was acquired from Carlo Erba Reagenti (Cornaredo MI, Italy). Phosphoric acid and potassium dihydrogen orthophosphate were obtained from Fisher Scientific (Loughborough, UK). Acetone, chloroform, and methanol were of analytical and HPLC grade.
Download the full study as PDF here: Enhanced stability and skin permeation of ibuprofen-loaded solid lipid nanoparticles based binary solid lipid matrix: Effect of surfactant and lipid compositions
or read it here
Thitirat Chantaburanan, Veerawat Teeranachaideekul, Anchalee Jintapattanakit, Doungdaw Chantasart, Varaporn Buraphacheep Junyaprasert, Enhanced stability and skin permeation of ibuprofen-loaded solid lipid nanoparticles based binary solid lipid matrix: Effect of surfactant and lipid compositions, International Journal of Pharmaceutics: X, Volume 6, 2023, 100205, ISSN 2590-1567,
https://doi.org/10.1016/j.ijpx.2023.100205.

