Impact of Tablet Size and Shape on the Swallowability in Older Adults
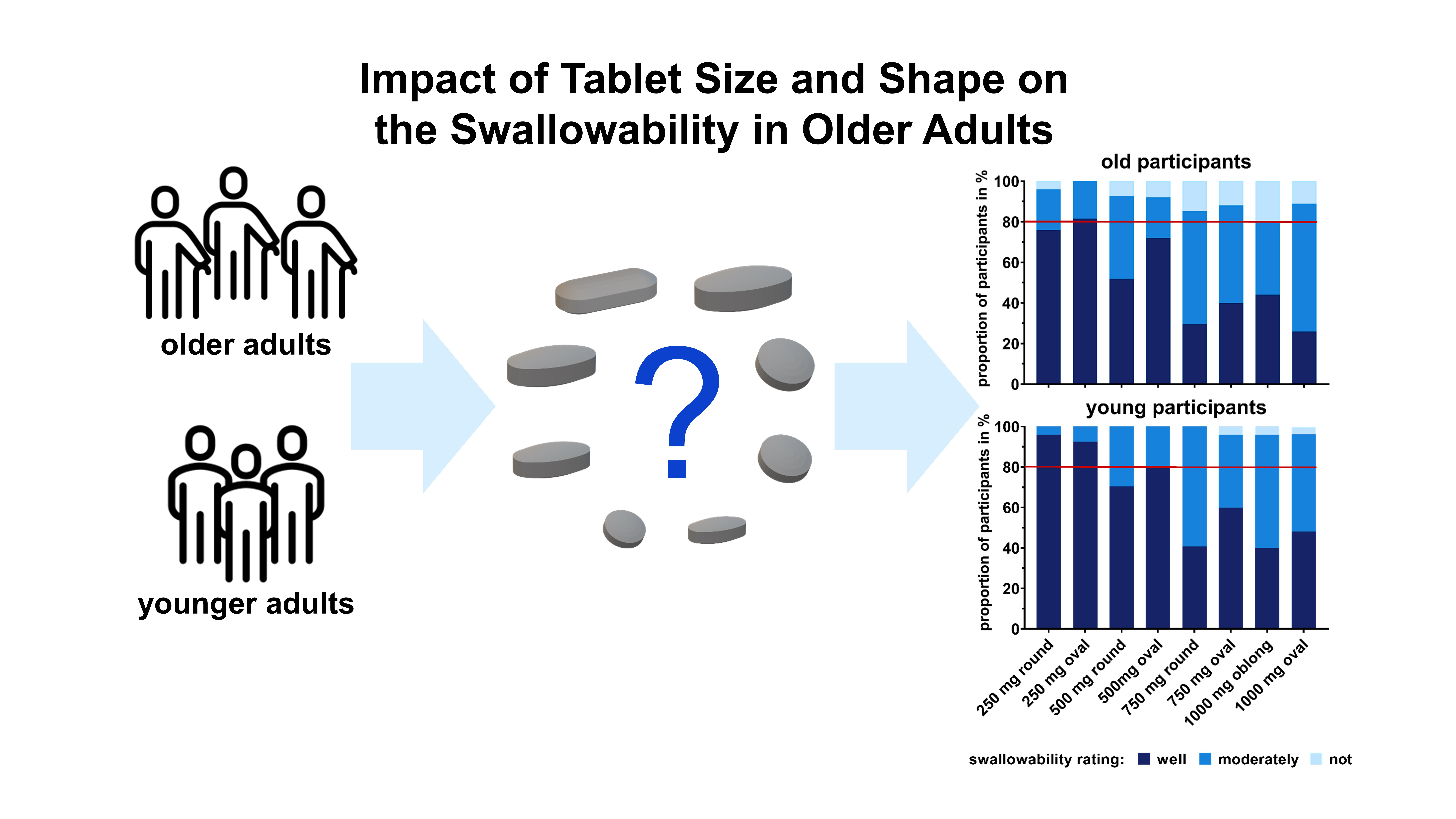
Older adults represent the major target population for oral medications, due to the high prevalence of multimorbidity. To allow for successful pharmacological treatments, patients need to adhere to their medication and, thus, patient-centric drug products with a high level of acceptability by the end users are needed. However, knowledge on the appropriate size and shape of solid oral dosage forms, as the most commonly used dosage forms in older adults, is still scarce. A randomized intervention study was performed including 52 older adults (65 to 94 years) and 52 young adults (19 to 36 years). Each participant swallowed four coated placebo tablets differing in weight (250 to 1000 mg) and shape (oval, round, oblong) in a blinded manner on three study days.
The choice of tablet dimensions allowed for a systematic comparison between different tablet sizes of the same shape, as well as between different tablet shapes. Swallowability was assessed using a questionnaire-based method. All tested tablets were swallowed by ≥80% of adults, independent of age. However, only the 250 mg oval tablet was classified as well swallowable by ≥80% of old participants. The same was true for young participants; however, they also considered the 250 mg round and the 500 mg oval tablet as well swallowable. Furthermore, swallowability was seen to influence the willingness to take a tablet on a daily basis, especially for an intake over longer time periods.
Download the full article as PDF here Impact of Tablet Size and Shape on the Swallowability in Older Adults
or read it here
Manufacturing of Placebo Tablets
Placebo tablets of different shape and size, as detailed in Table 1, were manufactured by direct compression using a Riva Piccola B-D tablet press (Riva G.B. Ltd., Aldershot, UK). Tablet cores consisted of 76.5% microcrystalline cellulose (Avicel® PH 102, DuPont Nutrition, Newark, DE, USA), 20.0% partially pregelatinized maize starch (Starch 1500®, Colorcon Inc., Harleysville, PA, USA), 0.5% hydrophilic fumed silica (Aerosil® 200 Pharma, Evonik Resource Efficiency GmbH, Essen, Germany), and 3.0% sodium stearyl fumarate (PRUV® Sodium Stearyl Fumarate, JRS Pharma GmbH & Co. KG, Rosenberg, Germany). The tablet cores were coated with a white polyvinyl alcohol-based coating (Opadry® II Complete Film Coating System 85F18422 White, Colorcon Inc., USA) on a Glatt GMPC 1 Mini coater (Glatt, Binzen, Switzerland). The target tablet core weight and the target coating amount of 2.5 mg/cm2 was reached by ±5%. The consistent coating thickness for all tablets ensured constant surface characteristics during the deglutition process. Disintegration of the different tablets was recorded to be between 4.6 and 13.8 min.
Hummler, H.; Stillhart, C.; Meilicke, L.; Grimm, M.; Krause, E.; Mannaa, M.; Gollasch, M.; Weitschies, W.; Page, S. Impact of Tablet Size and Shape on the Swallowability in Older Adults. Pharmaceutics 2023, 15, 1042. https://doi.org/10.3390/pharmaceutics15041042

