Formulation development of nanostructured lipid carrier-based nanogels encapsulating tacrolimus for sustained therapy of psoriasis
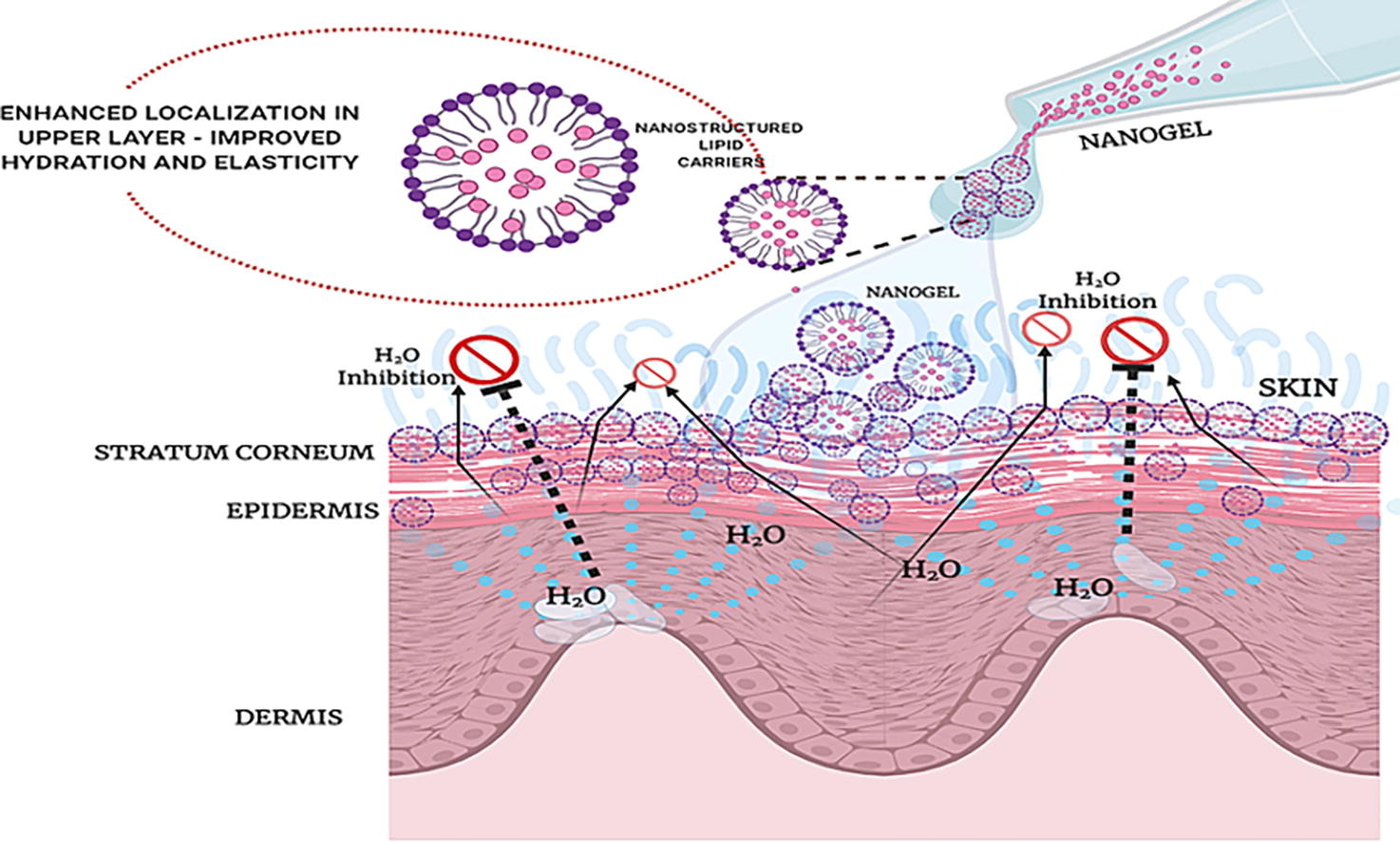
The goal of this study was to formulate tacrolimus nanogel based on nanostructured lipid carrier (NLC) in order to improve the efficacy, aesthetic, and patient compliance for the treatment of psoriasis. The microemulsion method was used to create phase diagrams and NLCs were prepared using points obtained from the microemulsion region and characterized. The gelling agent carbopol was used to develop an NLC-based nanogel. The pH, drug assay, viscosity, spreadability, and in vitro release of the nanogel, were evaluated.
Highlights
- Manuscript Focus: Developed stable and scalable tacrolimus nanogel for targeted drug delivery using nanostructured lipid carriers.
- Key Finding: Nanogel achieved 3X higher drug deposition, aiding psoriasis skin restoration effectively.
- Notable Outcome: Nanogel exhibited cytokine regulation potential ensuring promising therapy for psoriasis management.
Ex vivo cytotoxicity of the formulation was assessed in murine fibroblast cells. Oxazolone and imiquimod models of psoriasis were used to assess the effectiveness of the nanogel. The NLCs exhibited a submicron particle size of 320 ± 10 nm, a low polydispersity index (<0.3), and a zeta potential of −19.4 mV. Morphological analysis revealed spherical nanoparticles with an encapsulation efficiency of 60 ± 3 %. The nanogel maintained a pH of 6.0 ± 0.5 and possessed a remarkable drug content of 99.73 ± 1.4 %. It exhibited pseudoplastic flow behaviour, ensuring easy spreadability, and demonstrated sustained drug release exceeding 90 % over a 24-hr period.
Ex vivo cytotoxicity assessments revealed that the nanogel was safe because no cell death was induced. Nanogel resolved psoriatic blisters, was non-irritating and improved skin elasticity. The favorable properties, safety profile, and remarkable efficacy show the potential of the nanogel as a patient-friendly and effective therapeutic option for psoriasis treatment.
Read more here
Materials
Tacrolimus (Concord Biotech, India) was provided as a kind gift by Alkem Laboratories Ltd. All reagents used in the study are of HPLC grade. Milli Q-water (Millipore, Bedford, MA, USA) was used in the study.
Gift samples of solid lipids and liquid lipids include stearic acid (SD-Fine, India), Softisan® 601 (IOI Oleo GmbH, Germany), Tefose®63, Tefose®1500, Monosteol®, Lauroglycol®90, Capryol®90 (Gattefosse, India), Brij®C20 (Loba Chemicals, India), Miglyol®812 (Sasol, India), Capmul®PG 12, Carbopol (Lubrizol).
Pratik Kakade, Vandana Patravale, Ajit Patil, John Disouza, Formulation development of nanostructured lipid carrier-based nanogels encapsulating tacrolimus for sustained therapy of psoriasis, International Journal of Pharmaceutics,
2024, 124172, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124172.
Read also our introduction article on Stearic Acid here:


