Solid dispersion technology with porous material – Telmisartan
1. Introduction
Solid dispersion is one of the most effective technologies to improve API characteristics such as solubility, stability, and compressibility. Recently developed small molecule APIs with the aim to higher therapeutic effects, tend to become poor soluble, as much as 70% or higher currently under development have this problem. Special formulations have been developed to solve this challenge. Spray drying and hot-melt extrusion are proven options as solid dispersion but, it is necessary to have experience and need know-how to reach adequate polymer and parameters. Another option is porous material having huge internal space can be applied to solid dispersion as API carrier. It is a simple and easy process using common equipment.
Tomita’s FLORITE is a macro porous calcium silicate with considerable liquid absorbency and compressibility, and it is already used for solid dispersion formulation with poor soluble APIs. This paper refers such actual solid dispersion technology with porous material in published patent and paper.
2. Original patent for improvement of solubility in telmisartan
Telmisartan(4′-[1(1,4′-Dimethyl-2′-propyl[2,6′-bi-1H-benzimidazol]-1′-yl)methyl]-[1,1′-biphenyl]-2-carboxylic acid) is a medicine developed as angiotensin II receptor blocker for treatment of hypertension by Boehringer Ingelheim(BI). It is classified to BCS class II with high permeability and low solubility characteristics, and practically insoluble in water, sparingly soluble in strong acid and soluble in strong base.
Therefore, BI figured out specific formulation in their patent WO 2004/028505 to improve the solubility. They discovered a complex of telmisartan with surfactant or emulsifier works for increasing solubility of telmisartan. Telmisartan and surfactant or emulsifiers are dissolved into water under strong alkaline condition and mixed uniformly. The solution is served to granulation with saccharides which are described as water-soluble diluent in the patent, and telmisartan revert to solid form together with surfactant or emulsifier through the process.
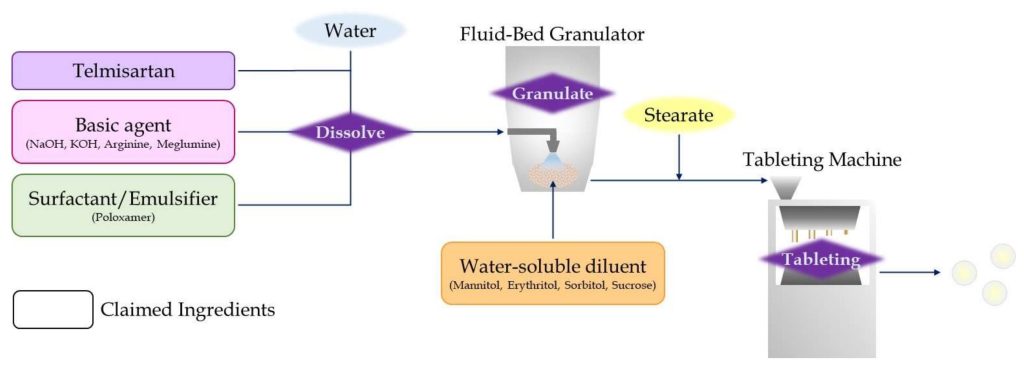
This achieves granules coated with a uniform complex of telmisartan, surfactant or emulsifier. Saccharides would be expected to support quick dissolution of the telmisartan layer as a soluble seed core. The encapsulated granules show improved solubility 10 to 20% compared to the absence of surfactant or emulsifier, in the patent. This patent was filed in a lot of countries and is still effective now in EU, Japan, Mexico, India, Korea, New Zealand, Philippine, Poland, and Russia.
3. Alternative patent for generic formulation of telmisartan
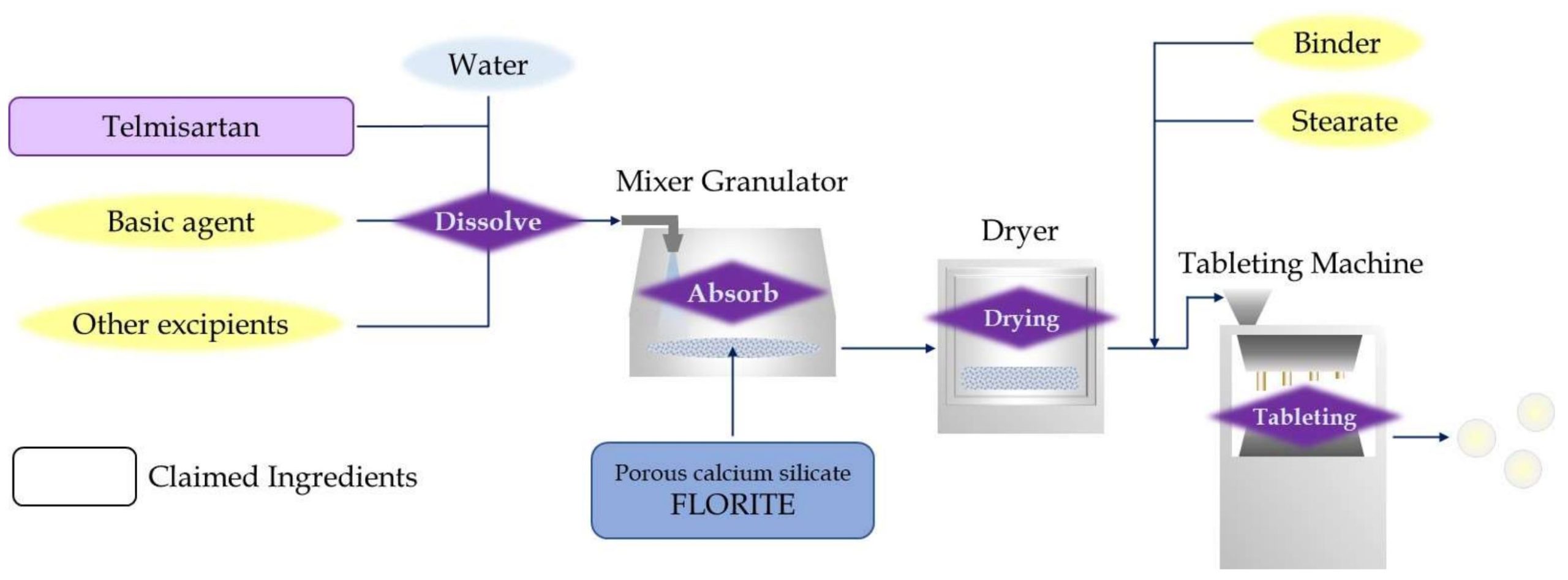
Kyorin Rimedio(KR), a Japanese generic pharmaceutical company, developed alternative formulation of telmisartan without surfactant and emulsifier to avoid patent conflict with BI, and it was patented as JP2015-67608 in Japan. KR has supplied the generic telmisartan medicine in Japanese market based on the patent. In their patent, telmisartan is absorbed or coated to porous inorganic excipient, and it achieves solid dispersion complex with improved solubility.
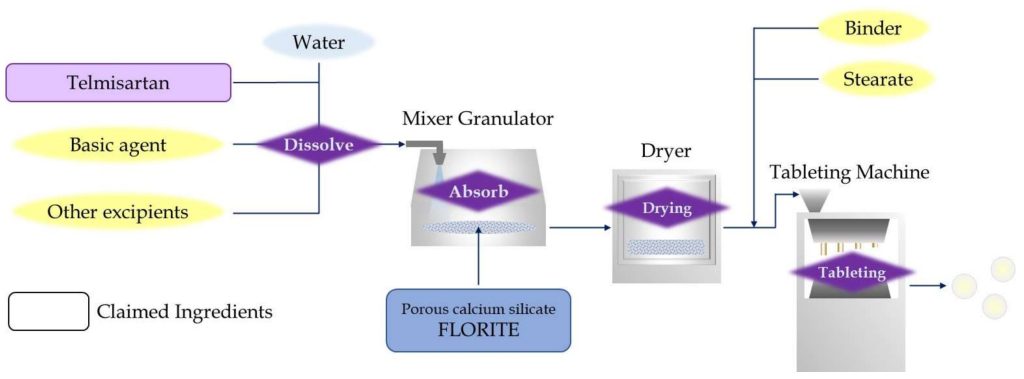
Telmisartan is dissolved into water, preferably under strong alkaline condition, and the solution is absorbed into calcium silicate. This is described as porous inorganic excipient in the patent, with conventional granulation process. The telmisartan is considered to exist in pores of calcium silicate and reverts to solid form when evaporate the water, forming a solid-dispersion complex. Telmisartan is micronized into nano-size by solid dispersion, and improved solubility due to increasing total surface area. Tomita’s FLORITE is mentioned and designated as suitable calcium silicate for solid dispersion in the patent.
The tablet made from the solid dispersion complex shows 100 times solubility compared to direct compression tablet with telmisartan, and improved solubility significantly. Similar telmisartan formulations with calcium silicate have been developed later in other Asian countries, that generic products rapidly spread there.
4. Sealed heated method of ethenzamide
Another solid dispersion technology with porous calcium silicate, with a sealed heated method was reported by Hanawa et al*. In their study, a solid dispersion complex of porous calcium silicate and ethenzamide was prepared in heated sealed container. Ethenzamide is absorbed into calcium silicate and transformed to amorphous form with improved solubility. This is one of the hot-melt extrusions, but unlike existing technologies, it does not require polymer. Tomita’s FLORITE was used in their study and is described as suitable API carrier with amorphization capability in the paper.
See the full White Paper on “Telmisartan” here
(click the picture to download the white paper)
Source: Tomita, White Paper “Telmisartan”
Do you need more information or a sample of Tomita excipients?


