Development of concentration prediction models for personalized tablet manufacturing using near-infrared spectroscopy
Abstract
In this work, concentration prediction models applicable to a very wide range of concentrations for personalized tablet manufacturing were developed using near-infrared (NIR) spectroscopy. Tablet manufacturing experiments were conducted, and NIR spectral data of the tablets were obtained. To search for an appropriate development method for the prediction models applicable to a very wide range of concentrations, three typical active pharmaceutical ingredients (APIs) were selected and two modeling methods were applied, namely, partial least squares (PLS) regression and extreme gradient boosting (XGBoost). The prediction models developed using the two methods were evaluated according to widely used criteria. The results showed that the model accuracy obtained using PLS regression was higher than that obtained using XGBoost. The modeling method using PLS regression was also applied to other typical APIs and inorganic compounds. These results indicate that the model accuracy was affected by the heat resistance of the compound. In addition, this modeling method could be useful in developing prediction models for ion-binding inorganic compounds and in suggesting the presence of metallic foreign matter in tablets.
Introduction
Personalized medicine is a promising new concept for dealing with challenges in health-care systems through patient-tailored treatment approaches (Cesario et al., 2021). By enabling patients to receive earlier diagnoses and optimal treatments, personalized medicine holds promise for improving health care (e.g., reducing side effects; Vogenberg et al., 2010).
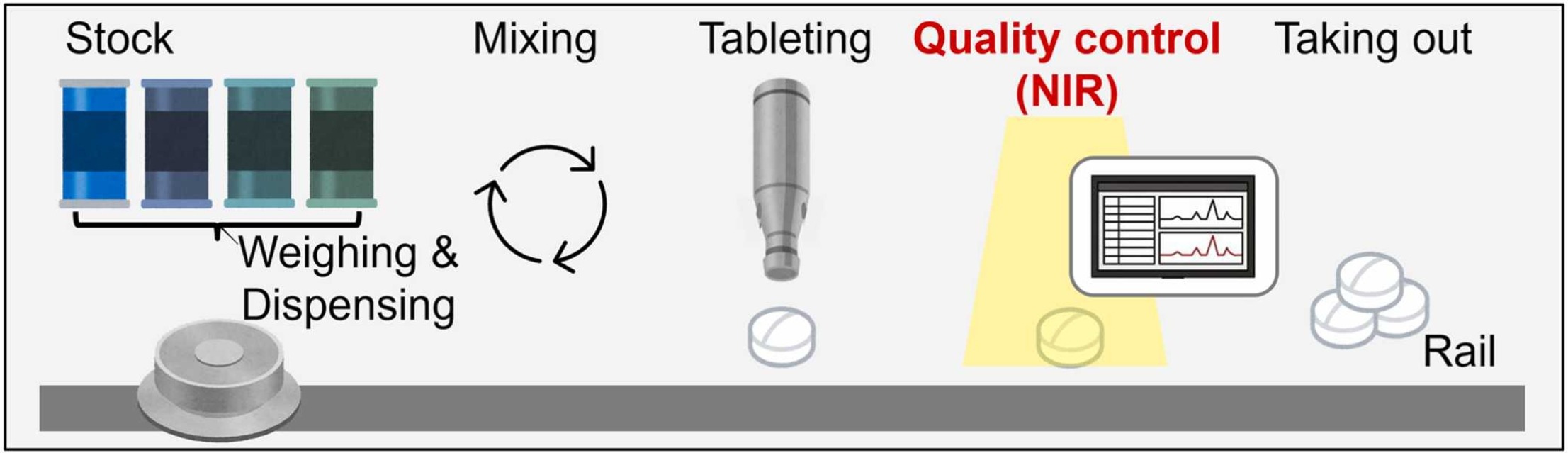
Tablets are generally mass-produced by pharmaceutical companies, but personalized tablet manufacturing has recently been attracting more attention because of the advantages mentioned above. Içten et al. (2015) presented a supervisory control strategy to achieve the critical quality attributes of solid oral dosages. Azad et al. (2018) developed a compact, portable, reconfigurable, and automated system for on-demand tablet manufacturing. Gültekin et al. (2021) applied three-dimensional (3D) printing, which is an additive manufacturing technology for personalized tablet manufacturing. Sundarkumar et al. (2022) proposed a novel unit operation to achieve the desired integration. However, the scope of these previous studies has been limited to process control or manufacturing, and the quality control methods for personalized tablet manufacturing are still in their infancy.
Quality control methods for personalized tablet manufacturing should be applicable for nondestructive, real-time, and full inspections, and be able to deal with a wide range of concentrations. There are two candidate methods for quality control in personalized tablet manufacturing: near-infrared (NIR) spectroscopy and Raman spectroscopy. NIR spectroscopy is unlikely to damage the tablet components because the energy of NIR light is low (Zou et al., 2012). The spectroscopy can also reflect physical conditions such as particle size and density (Cen and He, 2007). By contrast, Raman spectroscopy is useful for substance identification because the Raman spectra present a sharp peak (Logaranjan et al., 2016). However, the light intensity used in Raman spectroscopy is higher than that used in NIR spectroscopy (Downes, 2019), and is more likely to decompose pharmaceutical heat-sensitive ingredients in the tablets. Therefore, in this work, we focused on NIR spectroscopy.
Several authors have developed quality control methods for tablet manufacturing using NIR spectroscopy. Broad et al. (2001) developed calibration models applicable to a specific range (i.e., from 2.94 wt% to 17.64 wt%) for intact steroid tablets using NIR spectroscopy. Blanco and Peguero (2010) performed an analysis of pharmaceuticals using NIR spectroscopy without a reference method. Blanco et al. (2010) demonstrated the usefulness of NIR spectroscopy for product quality assessment. De Leersnyder et al. (2018) proposed an in-line NIR method with high-speed acquisition of NIR spectra to allow real-time release. Román-Ospino et al. (2016) presented NIR spectroscopic calibration models for the real-time prediction of powder density. Gosselin et al. (2018) proposed an automatic sorting system capable of testing every ibuprofen tablet without creating a bottleneck in the production line. Biagi et al. (2021) constructed a quantitative NIR analytical method for in-line quantitative analysis of bilastine in a powder mixture during the mixing process. In mass-produced tablet manufacturing, only a predetermined concentration has been handled, but in personalized tablet manufacturing, a very wide range of concentrations (e.g., from 0 wt% to 100 wt%) should be covered. However, prediction models applicable to a very wide range of concentrations have yet to be developed.
This work deals with the development of concentration prediction models applicable to a wider range of concentrations for personalized tablet manufacturing using NIR spectroscopy. Tablet manufacturing experiments were performed, and NIR spectrum data of the tablets were obtained. To identify an appropriate development method for the prediction models applicable to a wide range of concentrations, three typical active pharmaceutical ingredients (APIs) were selected and two modeling methods were applied: partial least squares (PLS) regression (Wold, 1985) and extreme gradient boosting (XGBoost; Chen and Guestrin, 2016), which is one of the gradient boosting decision tree methods. The prediction models developed using the two methods were evaluated according to criteria that are widely applied in the pharmaceutical industry. The modeling method that was regarded as appropriate was also applied to predict concentrations of other typical APIs and inorganic compounds, and detect metallic foreign matter in tablets.
Read more here
Materials
The materials used in this study are listed in detail in Table 1. Methacrylic acid copolymer LD (Evonik Nutrition & Care GmbH, Germany), ethenzamide (Iwaki Seiyaku Co., Ltd., Japan), and anhydrous caffeine (Shizuoka Coffein Co., Ltd., Japan) were adopted to search for an appropriate development method for prediction models applicable to a wide range of concentrations (Section 3). Ibuprofen (BASF Japan Ltd., Japan), acetaminophen (Yamamoto Corporation Co., Ltd., Japan), granfiller (Daicel).
Yusuke Hayashi, Saho Okazaki, Kensaku Matsunami, Kazuya Tanabe, Takuya Nagato, Hirokazu Sugiyama, Development of concentration prediction models for personalized tablet manufacturing using near-infrared spectroscopy, Chemical Engineering Research and Design, 2023, ISSN 0263-8762, https://doi.org/10.1016/j.cherd.2023.10.009.
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


