Viatel™ Bioresorbable Polymers – by Ashland
Ashland is a premier global specialty chemicals company serving customers in a wide range of consumer and industrial markets. With the Viatel™ platform, Ashland offers five families of bioresorbable polymers for parenteral controlled release drug delivery systems and medical devices.
viatel™ bioresorbable polymers are useful in the delivery of many drugs, including small molecules, peptides, proteins, vaccines, and other biomolecules. Scientists use viatel™ polymers in formulation strategies to produce controlled release injectable depots, micro/nano particles and solid implants that provide sustained release of the drug, from days to months.
For drug delivery, Ashland offers amorphous homopolymers and copolymers, including:
- Poly(D, L-lactide) (PDLLA) and
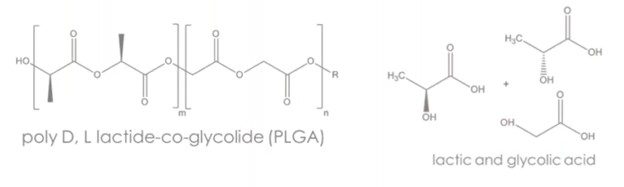
- Poly(D, L-lactide-co-glycolide) (PLGA)
For medical devices, Ashland offers semi-crystalline and amorphous homopolymers and copolymers including:
- Poly(L-lactide) (PLLA),
- Poly(ɛ-caprolactone) (PCL) and
- Poly(L-lactide-co-ɛ-caprolactone) (PLCL).
All Ashland Viatel™ bioresorbable polymers can be custom produced with defined chemical structures, molar masses (molecular weight or inherent viscosity) and selective terminal end groups.

In October 2023, Ashland introduced Viatel™ Ultrapure high-purity bioresorbable polymers as the result of Ashland’s commitment to continuous improvement in response to customer needs.
Viatel™ Ultrapure polymers are high-purity, controlled-release polymers that provide improved release consistency and extended-release durations; they are better suited for sensitive drug compounds in long-acting injectables and implants (LAII).
Removing residual monomer reduces acidic equivalents and results in a more consistent rate of water uptake and degradation kinetics. This means more reproducible performance while also creating a more neutral pH environment. viatel™ Ultrapure polymers leverage a proprietary purification process that reduces total residual monomer content specifications to below 0.5% with typical batch results of approximately 0.1%. Furthermore, these polymers are pre-filtered during the purification process to ensure exceptional quality.
These low-monomer products are available as GMP grades across the Viatel™ platform and provide formulators greater versatility when solving challenging formulation problems.
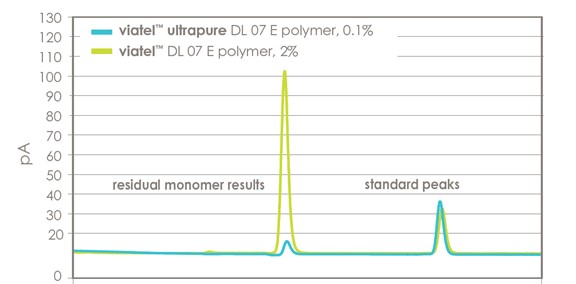
low residual monomer
Figure 1 displays the gas chromatography comparison for Viatel™ Ultrapure polymer grades versus Viatel™ standard grades and shows significantly lower residual monomer content.
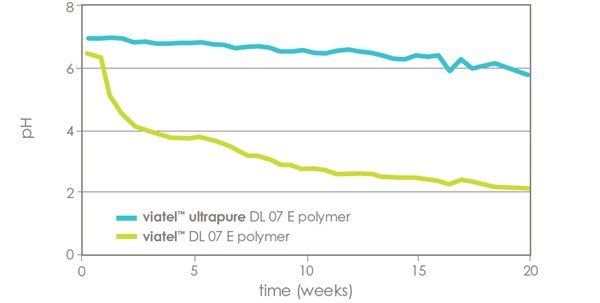
reduced acidity
Figures 2 shows change in pH over time to a phosphate buffer saline (PBS) solution containing Viatel™ polymers or their Viatel™ Ultrapure polymer equivalents. Viatel™ Ultrapure polymers exhibit less acidity over time compared to their standard counterparts.
improved stability
Reduced acidity can preserve sensitive APIs, as demonstrated in figure 3. Omeprazole was dissolved in N-Methyl-2-pyrrolidone (NMP) and exposed to Viatel™ polymer or the equivalent Viatel™ Ultrapure polymer. Viatel™ Ultrapure polymer demonstrated reduced degradation to the API.
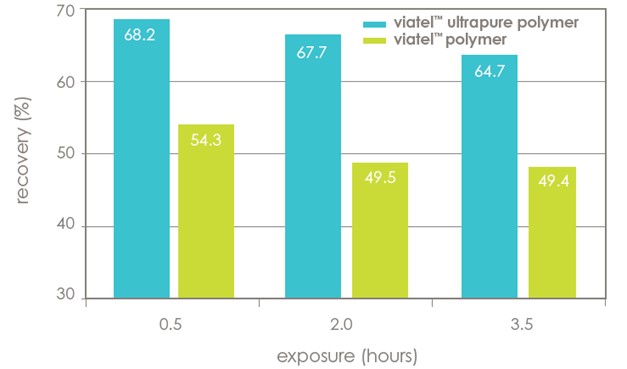
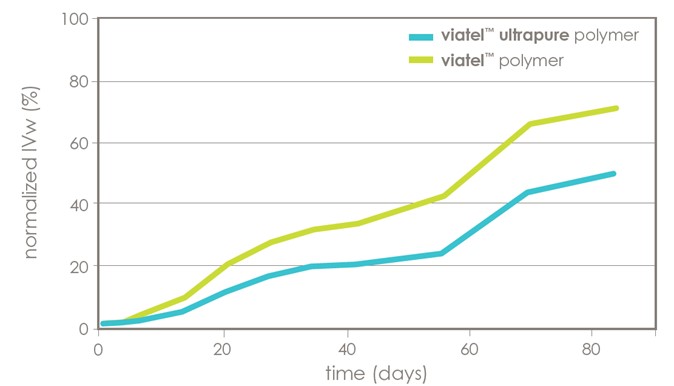
extended release using viatelTM ultrapure polymer
Hot melt extrusion was utilized to fabricate two implants consisting of metformin (10% drug load) and either viatel™ DLG 7509 E polymer or viatel™ Ultrapure DLG 7509 E polymer. These implants were exposed to phosphate buffered saline at 37 °C for 5 weeks, sampling at various timepoints to assay for molecular weight and drug released. viatel™ standard grade polymer experienced a greater loss of molecular weight due to acid catalyzed hydrolysis of the polymer, which resulted in a faster release profile compared to viatel™ Ultrapure polymer, as shown in figure 4.
manufacturing and quality
Viatel™ bioresorbable polymers are produced in an ISO 14644-1 Class 8 cleanroom environment and comply with USP/NF General Chapter <1078> Good Manufacturing Practices for Bulk Pharmaceutical Excipients and The Joint IPEC-PQG Good Manufacturing Practices Guide for Pharmaceutical Excipients.
Ashland holds a type IV excipient drug master file (DMF) with the FDA for Viatel™ bioresorbable polymers (DMF number 33847) and holds a China Excipient DMF with National Medical Products Administration (NMPA) for PLGA 5050, PLGA 7525 and PLGA 8515. During 2020-2024, Ashland manufactures these materials at a state-of-the-art GMP manufacturing and R&D facility located in Mullingar, Ireland.
Download the full viatel ultrapure sell sheet here: Viatel Ultrapure Sell Sheet

See the article at Ashland
Interested in learning more about long-acting injectables? View our webinar with Oakwood labs showcasing viatel polymers in scale-up developments :
Long-Acting Injectables (LAI): Microsphere Design, Development and Scale-up – Xtalks
Source: Ashland, website www.ashland.com/industries


