Encapsulating cannabinoids in lipid-based nanoparticles: CBD vs. CBDA
Enhancing cannabinoids’ therapeutic efficacy
Cannabinoids have a long history in medicine given their therapeutic effects (1). However, due to their lipophilic nature, they are poorly absorbed by the digestive system which hinders their potential as therapeutics (2).
Nanoparticles offer a solution to this problem. By encapsulating these hydrophobic molecules into water miscible vehicles, they can be better absorbed by the digestive system, thus enhancing their therapeutic effect (3).
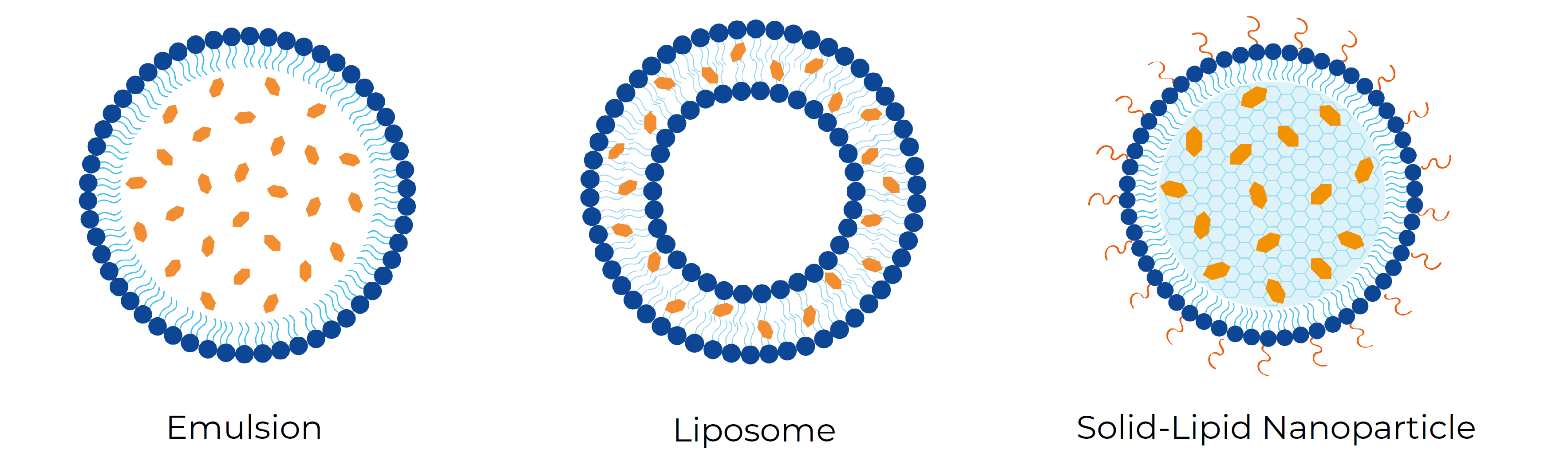
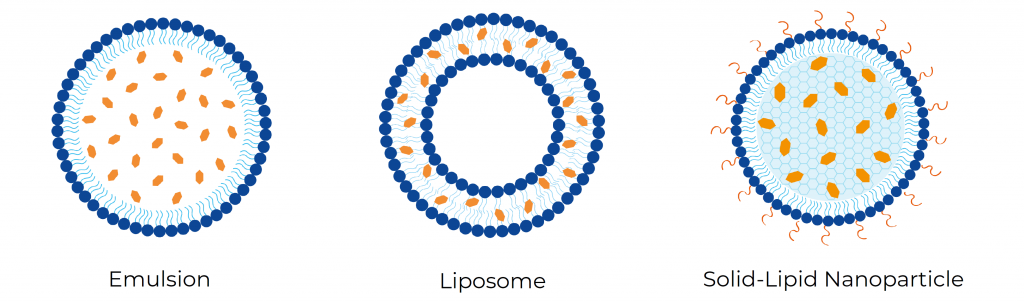
At Ascension Sciences, we have explored the different types of nanoparticles to encapsulate cannabinoids. Specifically, lipid-based formulations have been successfully optimized for the encapsulation of THC and CBD. One of the next steps in our research and development explorations was to use these optimized parameters to encapsulate minor cannabinoids such as CBN, CBC, CBG, CBDA and THCA into three types of lipid-based nanoparticles: emulsions, liposomes and solid-lipid nanoparticles (SLNPs) – Figure 1.
CBD vs. CBDA
This technical note presents the comparison between the encapsulation of CBD vs. CBDA using formulation parameters that were previously optimized for CBD. The goal of the study was to provide insights into the differences of the two cannabinoids and to describe the beginnings of an optimization methodology where one lead formulation concept can become the starting point for an alternate active ingredient and development project.
OVERVIEW
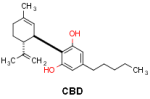
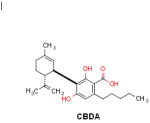
CBDA is suggested to have similar therapeutic benefits as CBD (4). CBDA is synergically working with CBD and might be responsible for the entourage effect (5). CBDA, like other minor cannabinoids which are found at lower concentrations in cannabis, has shown various medicinal benefits like anticonvulsant and antiemetic activity (4,6). CBDA is the precursor acidic form of CBD and can be converted into CBD by decarboxylation.
In this study, we compared encapsulation efficiency and nanoparticle stability of each cannabinoid formulation. Encapsulation efficiency is the amount of cannabinoid that is encapsulated into the nanoparticle upon formulation. Stability is determined by measuring the size and PDI every 7 days for a month of storage at different conditions to establish changes in the particle. A stable nanoparticle does not significantly change in size and PDI remains below 0.2, meaning that the particle population is uniform in size and there is no aggregation of the particles over time. Lastly, we determined the cannabinoid retention, which is the amount of cannabinoid that remains inside the nanoparticles after 30 days of storage.
For the purpose of this article, we will focus on the nanoparticles stored at 4°C.
METHODS
The formulation parameters for each nanoparticle type can be found in Table 1.
Each formulation type was used on either CBD or CBDA using the same formulation parameters.
All of the nanoparticle formulations were mixed using a low energy approach achieved by the NanoAssemblr Benchtop microfluidic instrument from Precision Nanosystems.
| Emulsion | Liposome | SLNP | |
| Lipid composition | TweenⓇ 80 : SpanⓇ 80 : Hemp Seed Oil | POPC : Chol : DSPE-PEG2000 | Chol : POPC : DSPE-PEG2000 |
| Organic solvent | Ethanol | ||
| Aqueous solvent | Deionized water | PBS pH 7.4 | |
| Solvent removal | Dialysis in aqueous media | ||
| Lipid:Cannabinoid | 10:1 | ||
Table 1: Formulation parameters for the different types of nanoparticles
RESULTS
Encapsulation efficiency (EE%)
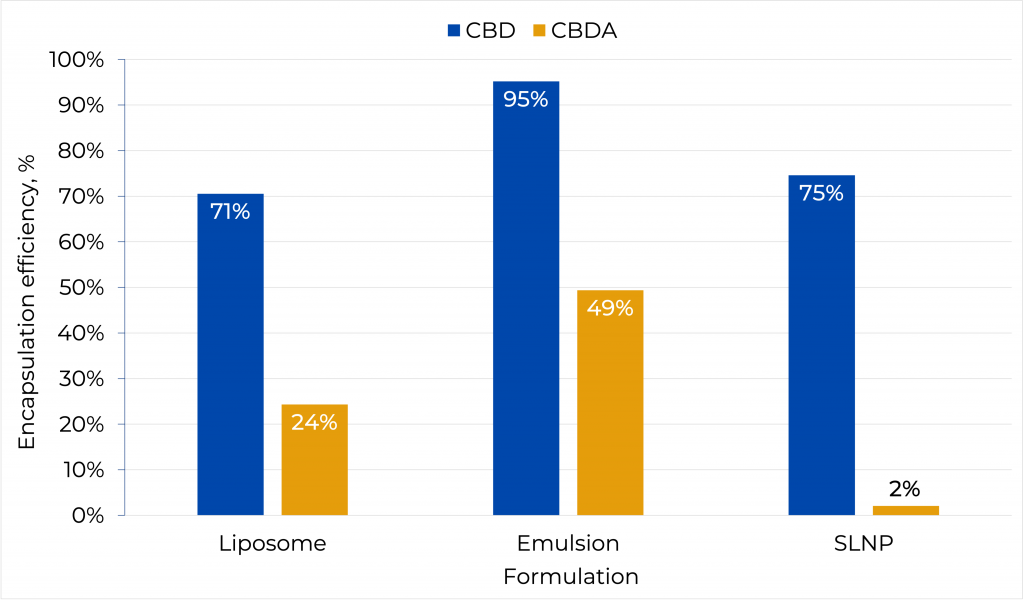
| Type of nanoparticle | CBD concentration, mg/mL | CBDA concentration, mg/mL |
| Liposome | 0.88 | 0.35 |
| Emulsion | 3.98 | 2.21 |
| SLNP | 0.31 | 0.01 |
* CBD solubility in water: 0.1 ug/mL (7)
Table 2: Encapsulated cannabinoid concentration
- Encapsulation efficiency for CBD in all the particle types is significantly greater than for CBDA formulations.
- CBD emulsion nanoparticles show the highest encapsulation within the three types of nanoparticles.
- All the nanoparticles allow a higher concentration of the cannabinoids in an aqueous solution compared to the free drug.
Nanoparticle stability over 30 days of storage at 4°C
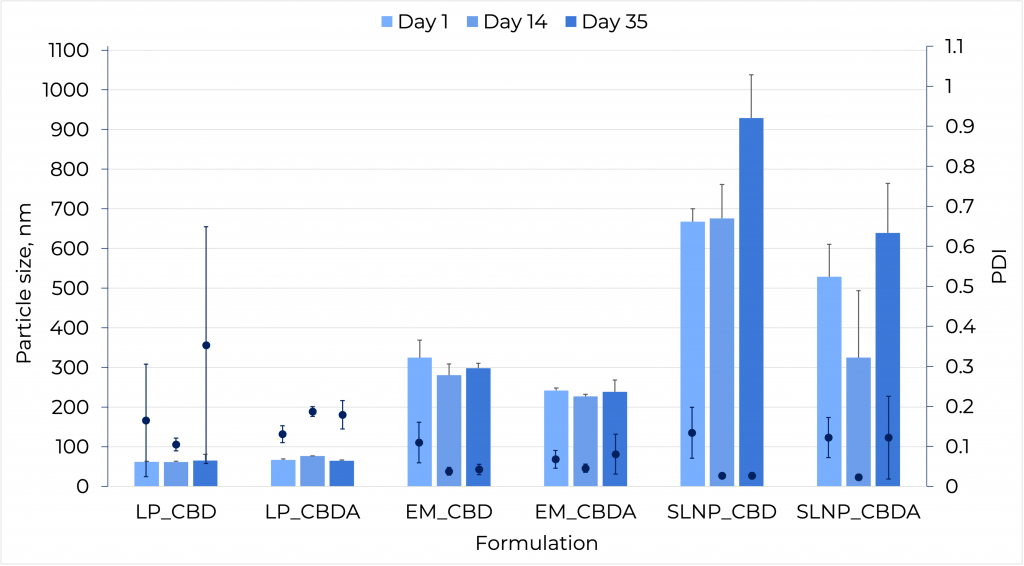
- All PDI values were below 0.2, except for the CBDA liposome at day 35, which indicates that there was no aggregation of the particles and the particle population of the specific size remained stable.
- The PDI for the CBDA liposome at day 35 increased above 0.2 which indicates that the particles are aggregating.
- Particle size and PDI were stable for both CBD and CBDA liposomes and emulsions.
- Liposomes have the lowest particle size within the three types of nanoparticles, 60 +/- 3 nm, and are the same for both CBD and CBDA.
- Emulsions show a particle size of 300 +/- 20 nm for both CBD and CBDA.
- SLNPs show an increase in particle size of both CBD and CBDA SLNPs. CBD SLNPs show a 43% increase in particle size and CBDA show 21% increase compared to the day the samples were formulated.
Cannabinoid retention over 35 days of storage at 4°C

- All CBD particles had higher than 50% retention over 35 days of storage in aqueous solution at 4°C, the highest retention being for the SLNPs.
- The CBDA liposomes showed 64% retention while the emulsions and SLNPs did not retain any cannabinoid inside the nanoparticles.
CONCLUSION
One formulation does not fit all cannabinoids
Even though the size and PDI across the different types of nanoparticles does not vary significantly between CBD and CBDA, the encapsulation efficiency and drug retention results show that the formulations optimized for CBD are not suitable for CBDA. This was an unexpected result given how similar these two compounds are in terms of structure. The expectation was that the lead CBD formulations would, at the very least, present a reasonable starting point for CBDA formulation development. However, looking at the encapsulation efficiency and CBDA retention, it is clear that the presence of the carboxylic group has a significant effect on the outcome of the formulation.
A possible explanation for the observed differences is that CBDA interacts differently with the phospholipids and surfactants that form the different lipid-based nanoparticles given its acidic group. Thus, even though these two compounds are very similar in structure, their physical properties and how they interact with other molecules is different due to the presence of the carboxylic group in CBDA.
FUTURE STUDIES
ASI aims to develop and optimize a formulation with different excipients, possibly ionizable lipids or a different buffer media at a different pH, to encapsulate CBDA and other minor cannabinoids.
Find out more about Ascension Sciences here!
REFERENCES
- Malmo-Levine D. Holland J. The Pot Book: A Complete Guide to Cannabis. Rochester, Vermont: Park Street Press; 2010
- Bruni, N. et al. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 23, 2478 (2018).
- Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011;12:62–76.
- Pertwee, R. G. et al. Cannabidiolic acid methyl ester, a stable synthetic analogue of cannabidiolic acid, can produce 5‐HT1A receptor‐mediated suppression of nausea and anxiety in rats. Br. J. Pharmacol. 175, 100 (2018).
- Wakshlag, J. J. et al. Pharmacokinetics of Cannabidiol, Cannabidiolic Acid, Δ9-Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Related Metabolites in Canine Serum After Dosing With Three Oral Forms of Hemp Extract. Front. Vet. Sci. 7, 505 (2020).
- Russo, E. B. Cannabis therapeutics and the future of neurology. Front. Integr. Neurosci. 12, (2018).
- Mannila J, Järvinen T, Järvinen K, Jarho P. Precipitation complexation method produces cannabidiol/beta-cyclodextrin inclusion complex suitable for sublingual administration of cannabidiol. J Pharm Sci. 2007;96(2):312-319

