2021 FDA approvals
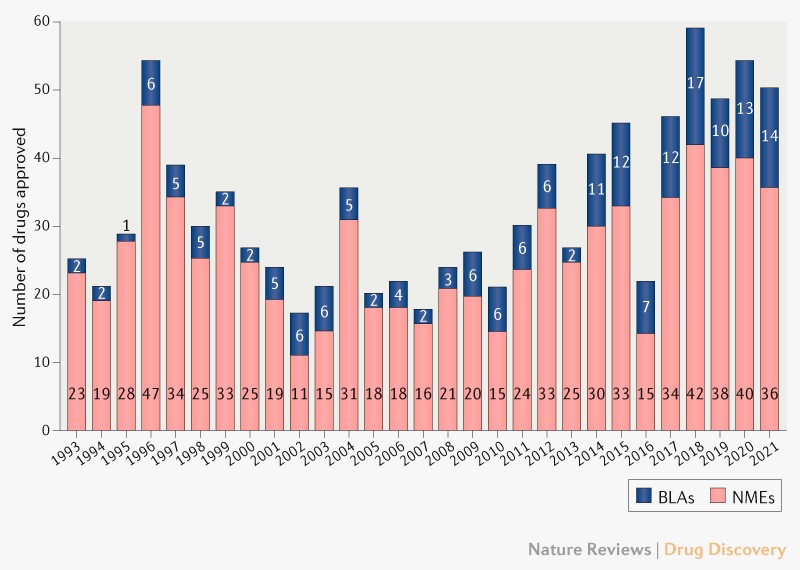
The FDA approved 50 novel drugs in 2021, including the first KRAS inhibitor for cancer and the first anti-amyloid antibody for Alzheimer’s disease.
Article by Asher Mullard – Nature Reviews Drug Discovery – 04 January 2022
The FDA’s approval count last year was in line with recent trends, despite the continued impact of COVID-19. The agency’s Center for Drug Evaluation and Research (CDER) approved 50 novel therapeutics in 2021, down from 53 in 2020 .The 5-year average sits at 51 drugs per year. A decade ago, it was 24 drugs per year.
TABLE 1 | CDER APPROVALS IN 2021
| Drug (brand name) | Sponsor | Properties | Indication |
|---|---|---|---|
| Vericiguat (Verquvo) | Merck & Co./Bayer | sGC stimulator | Chronic heart failure |
| Cabotegravir; rilpivirine (Cabenuva Kit) | ViiV | INSTI and an NNRTI | HIV-1 infection |
| Voclosporin (Lupkynis) | Aurinia | Calcineurin inhibitor | Lupus nephritis |
| Tepotinib (Tepmetko) | EMD Serono | MET kinase inhibitor | NSCLC |
| Umbralisib (Ukoniq) | TG Therapeutics | PI3Kδ and CK1ε inhibitor | MZL, follicular lymphoma |
| Evinacumab (Evkeeza)a | Regeneron | ANGPTL3-targeted mAb | HoFH |
| Trilaciclib (Cosela) | G1 Therapeutics | CDK4 and CDK6 kinase inhibitor | Chemotherapy-induced myelosuppression |
| Casimersen (Amondys 45) | Sarepta | Exon 45-skipping ASO | DMD |
| Fosdenopterin (Nulibry) | BridgeBio | cPMP | MoCD type A |
| Melphalan flufenamide (Pepaxto)b | Oncopeptides | Peptide-conjugated alkylating drug | Multiple myeloma |
| Dexmethylphenidate; serdexmethylphenidate (Azstarys) | Commave Therapeutics | CNS stimulant | ADHD |
| Tivozanib (Fotivda) | Aveo | VEGFR kinase inhibitor | Renal cell carcinoma |
| Ponesimod (Ponvory) | J&J | S1P receptor modulator | Relapsing multiple sclerosis |
| Dasiglucagon (Zegalogue) | Zealand Pharma | Glucagon receptor agonist | Severe hypoglycaemia |
| Viloxazine (Qelbree) | Supernus | SNRI | ADHD |
| Drospirenone; estetrol (Nextstellis) | Mayne Pharma | Spironolactone and oestrogen analogues | To prevent pregnancy |
| Dostarlimab (Jemperli)a | GlaxoSmithKline | PD1-targeted mAb | Endometrial cancer |
| Loncastuximab tesirine (Zynlonta)a | ADC Therapeutics | CD19-targeted ADC | B-cell lymphoma |
| Pegcetacoplan (Empaveli) | Apellis | Complement protein C3 inhibitor | PNH |
| Amivantamab (Rybrevant)a | J&J | EGFR×METR bispecific antibody | EGFR exon 20-mutated NSCLC |
| Piflufolastat F-18 (Pylarify) | Progenics | Radiolabelled PSMA imaging agent | Prostate cancer imaging |
| Infigratinib (Truseltiq) | BridgeBio | FGFR2 kinase inhibitor | FGFR2-mutated bile duct cancer |
| Sotorasib (Lumakras) | Amgen | KRAS-G12C inhibitor | KRASG12C-mutated NSCLC |
| Olanzapine; samidorphan (Lybalvi) | Alkermes | Atypical antipsychotic and opioid antagonist | Schizophrenia and bipolar I disorder |
| Ibrexafungerp (Brexafemme) | Scynexis | Triterpenoid antifungal | Vulvovaginal candidiasis |
| Aducanumab (Aduhelm)a | Biogen/Eisai | Amyloid-β-targeted mAb | Alzheimer’s disease |
| Asparaginase erwinia chrysanthemi (Rylaze)a | Jazz | Recombinant asparagine-specific enzyme | ALL and LBL, in patients allergic to E. coli-derived products |
| Finerenone (Kerendia) | Bayer | Non-steroidal MR antagonist | CKD with type 2 diabetes |
| Fexinidazole (Fexinidazole) | Sanofi/DNDi | Nitroimidazole antimicrobial | Sleeping sickness |
| Belumosudil (Rezurock) | Kadmon | ROCK2 kinase inhibitor | Chronic GVHD |
| Odevixibat (Bylvay) | Albireo | IBAT inhibitor | Pruritus in PFIC |
| Anifrolumab (Saphnelo)a | AstraZeneca | IFNAR-targeted mAb | SLE |
| Avalglucosidase alfa (Nexviazyme)a | Sanofi | Recombinant α-glucosidase | Pompe disease |
| Belzutifan (Welireg) | Merck & Co. | HIF-2α inhibitor | von Hippel-Lindau disease |
| Difelikefalin (Korsuva) | Cara Therapeutics | κ-Opioid receptor agonist | Pruritus associated with CKD |
| Lonapegsomatropin (Skytrofa)a | Ascendis Pharma | PEGylated human growth hormone | Growth failure due to GHD |
| Mobocertinib (Exkivity) | Takeda | EGFR kinase inhibitor | EGFR exon 20-mutated NSCLC |
| Tisotumab vedotin (Tivdak)a | Seagen/Genmab | Tissue-factor-directed ADC | Cervical cancer |
| Atogepant (Qulipta) | AbbVie | CGRP receptor antagonist | Episodic migraine |
| Maralixibat (Livmarli) | Mirum | IBAT inhibitor | Pruritus in Alagille syndrome |
| Avacopan (Tavneos) | ChemoCentryx | Complement 5a receptor antagonist | ANCA-associated vasculitis |
| Asciminib (Scemblix) | Novartis | ABL/BCR–ABL1 kinase inhibitor | Ph+ CML |
| Ropeginterferon alfa-2b (Besremi)a | Pharmaessentia | PEGylated interferon α-2b | Polycythaemia vera |
| Vosoritide (Voxzogo) | Biomarin | CNP analogue | Achondroplasia |
| Maribavir (Livtencity) | Takeda | CMV pUL97 kinase inhibitor | Post-transplant CMV infection |
| Pafolacianine (Cytalux) | On Target Labs | Fluorescent FR imaging agent | Ovarian cancer imaging |
| Efgartigimod alfa (Vyvgart)a | Argenx | FcRn-binding Fc fragment | Myasthenia gravis |
| Tezepelumab (Tezspire)a | Astrazeneca/Amgen | TSLP-targeted mAb | Severe asthma |
| Inclisiran (Leqvio) | Novartis/Alnylam | PCSK9-targeted siRNA | HeFH or ASCVD |
| Tralokinumab (Adbry)a | LEO Pharma | IL-13-targeted mAb | Atopic dermatitis |
aBiologic approval. bWithdrawn later in the year. A, accelerated; ADC, antibody–drug conjugate; ADHD, attention deficit hyperactivity disorder; ALL, acute lymphoblastic leukaemia; ASCVD, atherosclerotic cardiovascular disease; ASO, antisense oligonucleotide; B, breakthrough; CKD, chronic kidney disease; CMV, cytomegalovirus; CNP, C type natriuretic peptide; cPMP, cyclic pyranopterin monophosphate; DMD, Duchenne muscular dystrophy; FR, folate receptor; GHD, growth hormone deficiency; GVHD, graft-versus-host disease; HeFH, heterozygous familial hypercholesterolaemia; HoFH, homozygous familial hypercholesterolaemia; IBAT, ileal bile acid transporter; INSTI, integrase strand transfer inhibitor; LBL, lymphoblastic lymphoma; mAb, monoclonal antibody; MoDC, molybdenum cofactor deficiency; MR, mineralocorticoid receptor; MZL, marginal zone lymphoma; NSCLC, non-small-cell lung cancer; NNRTI, non-nucleoside reverse transcriptase inhibitor; O, orphan; P, priority; PEG, polyethylene glycol; Ph+ CML, Philadelphia chromosome-positive chronic myeloid leukaemia; PNH, paroxysmal nocturnal haemoglobinuria; S1P, sphingosine 1-phosphate; sGC, soluble guanylate cyclase; siRNA, small interfering RNA; SLE, systemic lupus erythematosus; SNRI, selective noradrenaline reuptake inhibitor; TSLP, thymic stromal lymphopoietin. Source: Drugs@FDA.
Further approvals from the FDA’s Center for Biologics Evaluation and Research (CBER) additions include a landmark mRNA vaccine and CAR-T cell products (Table 2). Emergency Use Authorizations (EUAs) made the news too, with antibodies and rapidly developed oral antivirals for COVID-19 (Table 3). CBER approvals and EUAs are not included in the annual new drug count, however.
Article information: Asher Mullard.

