Streamlining the development of an industrial dry granulation process for an immediate release tablet with systems modelling
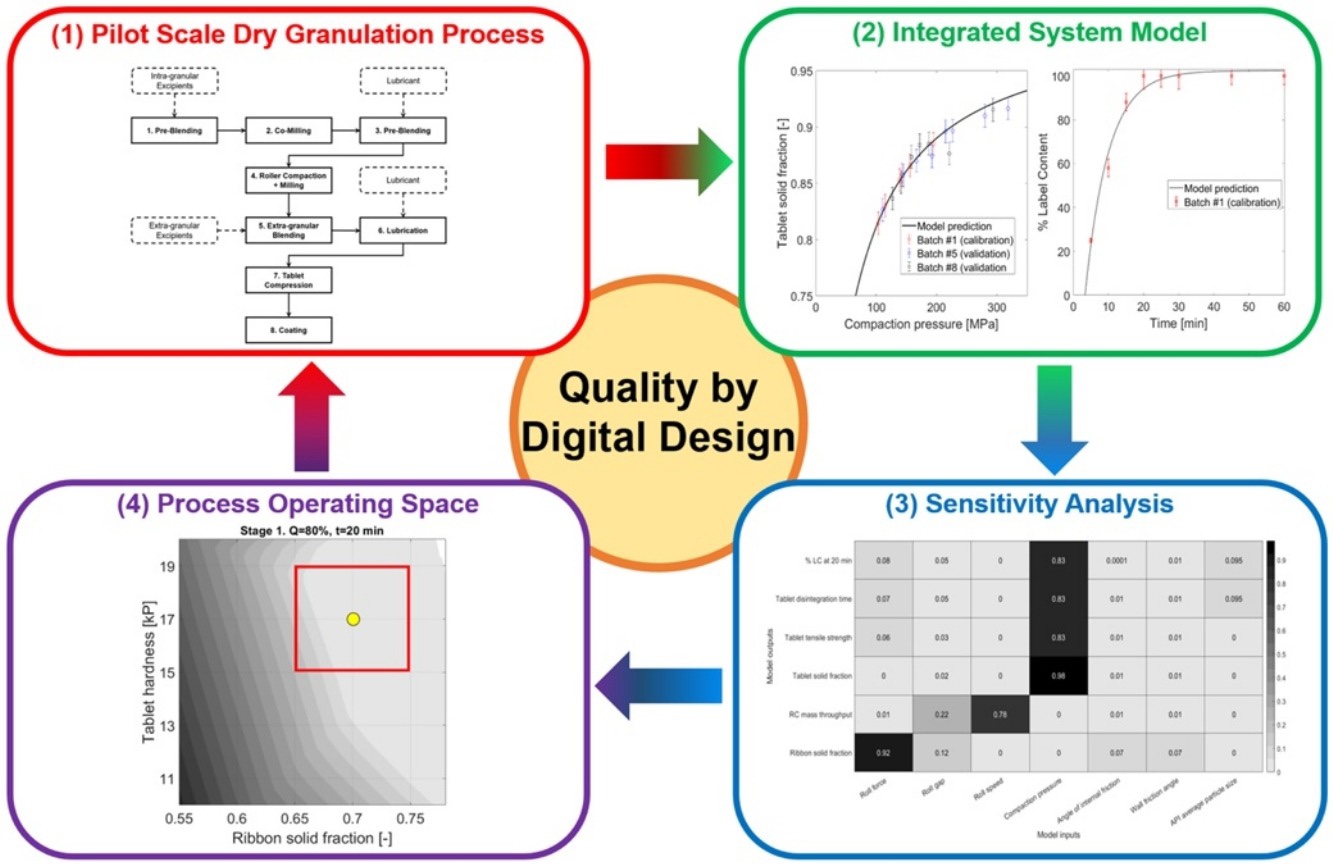
In industrial practice, the development of pharmaceutical dry granulation processes typically involves time- and resource-intensive multivariate experiments. These experiments are used to identify the set of operating conditions, their allowed ranges and chosen setpoints where the desired product quality and manufacturability criteria are met. The results are then used to define the control strategy for the manufacturing process to be included in the regulatory file.
In this study, we show how systems modelling can be used to streamline the development of an industrial dry granulation process for an immediate release tablet. We integrate existing and enhanced unit operation and product performance models with a Bayesian hierarchical model to predict the probability to meet the USP <711> dissolution test specifications, which represent the current standard in the pharmaceutical industry to demonstrate compliance with regulatory expectations.
We then use global sensitivity analysis to: (i) generate multivariate process understanding on the relative impact of material properties and process parameters on product quality attributes; (ii) predict the set of operating conditions (i.e., the process operating space) that allow us to meet the USP <711> test specifications with a given probability, as well as pre-defined manufacturability criteria. We finally use the results obtained at point (ii) to design targeted experiments to verify the predicted setpoints and operating space. We show how the proposed framework has the potential to remove >60% of the experimental burden (and hence the consumption of active pharmaceutical ingredient) required for process development compared to standard experimental protocols. Continue reading here
Gabriele Bano, Ranjit M. Dhenge, Samir Diab, Daniel J. Goodwin, Lee Gorringe, Misbah Ahmed, Richard Elkes, Simeone Zomer,
Streamlining the development of an industrial dry granulation process for an immediate release tablet with systems modelling,
Chemical Engineering Research and Design, 2021, ISSN 0263-8762,
https://doi.org/10.1016/j.cherd.2021.12.033.

