Lyophilized liposome-based parenteral drug development
Given the successful entry of several liposomal drug products into market, and some with decades of clinical efficacy, liposomal drug delivery systems have proven capabilities to overcome certain limitations of traditional drug delivery, especially for toxic and biologic drugs. This experience has helped promote new liposomal approaches to emerging drug classes and current therapeutic challenges.
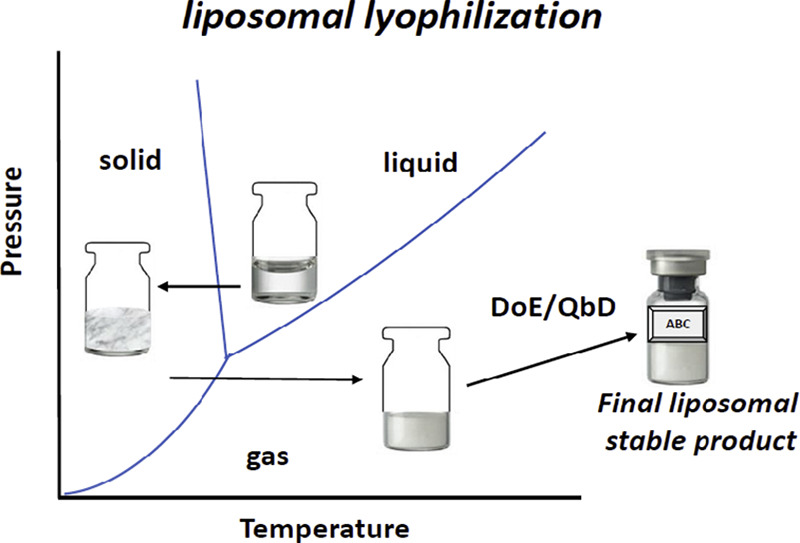
All approved liposomal dosage forms are parenteral formulations, a pathway demonstrating greatest safety and efficacy to date. Due to the intrinsic instability of aqueous liposomal dispersions, lyophilization is commonly applied as an important solution to improve liposomal drug stability, and facilitate transportation, storage and improve product shelf-life. While lyophilization is a mature pharmaceutical technology, liposome-specific lyophilization platforms must be developed using particular lyophilization experience and strategies.
This review provides an overview of liposome formulation-specific lyophilization approaches for parenteral use, excipients used exclusively in liposomal parenteral products, lyophilized liposome formulation design and process development, long-term storage, and current regulatory guidance for liposome drug products. Readers should capture a comprehensive understanding of formulation and process variables and strategies for developing parenterally administered liposomal drugs. Continue on liposomal drugs

