3D Printing of Dietary Products for the Management of Inborn Errors of Intermediary Metabolism in Pediatric Populations
Abstract
The incidence of Inborn Error of Intermediary Metabolism (IEiM) diseases may be low, yet collectively, they impact approximately 6–10% of the global population, primarily affecting children. Precise treatment doses and strict adherence to prescribed diet and pharmacological treatment regimens are imperative to avert metabolic disturbances in patients. However, the existing dietary and pharmacological products suffer from poor palatability, posing challenges to patient adherence. Furthermore, frequent dose adjustments contingent on age and drug blood levels further complicate treatment. Semi-solid extrusion (SSE) 3D printing technology is currently under assessment as a pioneering method for crafting customized chewable dosage forms, surmounting the primary limitations prevalent in present therapies. This method offers a spectrum of advantages, including the flexibility to tailor patient-specific doses, excipients, and organoleptic properties. These elements are pivotal in ensuring the treatment’s efficacy, safety, and adherence. This comprehensive review presents the current landscape of available dietary products, diagnostic methods, therapeutic monitoring, and the latest advancements in SSE technology. It highlights the rationale underpinning their adoption while addressing regulatory aspects imperative for their seamless integration into clinical practice.
Introduction
The term “Inherited Metabolic Disorder” (IMD) encompasses a diverse array of genetic disorders, where a deficiency in a specific enzyme, transporter, or regulatory protein disrupts normal metabolic pathways [1,2]. This enzymatic deficiency impedes the degradation of natural endogenous substrates, leading to their accumulation in various body tissues. Without appropriate treatment, these disorders can have fatal consequences. Given their genetic origin, IMDs are relatively rare, typically manifesting in children who display early symptoms, underscoring the critical importance of prompt diagnosis for timely intervention and the prevention of metabolic or severe multisystemic consequences. The estimated global birth prevalence of IMD stands between 50 and 125 per 100,000 live births [3], making them a significant contributor to pediatric mortality and morbidity worldwide. Although individually rare, collectively, these disorders represent a common health concern.
Inborn errors of intermediary metabolism (IEiM) constitute a substantial portion of IMDs, affecting the breakdown of low-molecular-weight nutrient compounds. This group includes 13 out of 24 categories in the current International Classification of IMD [1]. Main disorders within IEiM result from genetic defects in enzymes or cofactors involved in the metabolism of amino acids (e.g., phenylketonuria, maple syrup urine disease (MSUD), homocystinuria, tyrosinemias, organic acidemias, urea cycle disorders (UCDs)), carbohydrates (e.g., galactosemia, hereditary fructose intolerance, glycogen storage disease), and fatty acids (e.g., fatty acid β-oxidation defects) [4]. Disruptions in these pathways, often caused by enzyme alterations, can lead to toxic substance accumulation or energy production deficiency, which is detectable through specific biochemical markers. Newborn screening (NBS), implemented worldwide since the 1960s, has facilitated early diagnosis and treatment of IEiM [5,6,7].
IEiMs typically manifest as multisystemic diseases with both neurological and non-neurological symptoms, occasionally accompanied by distinctive physical features [8]. Characterized by a symptom-free neonatal period, signs of intoxication emerge during early childhood due to toxic compound accumulation. However, manifestations can also occur later, displaying intermittent, chronic, or progressive patterns leading to neurodegeneration [9]. Despite extensive efforts, the first-line treatment remains dietary control and nutritional supplementation. Administering treatment poses challenges for both patients and their caregivers, with doses often being prescribed on a trial-and-error basis.
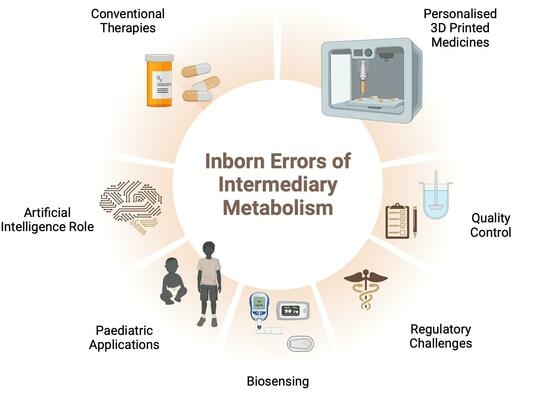
This review aims to offer an encompassing perspective on current dietary product therapies for pediatric IEiM patients, highlighting their advantages and disadvantages. Additionally, it explores recent innovations in three-dimensional (3D) printing technology, emphasizing its potential in personalizing pediatric medication for improved adherence, palatability, and dose customization. The use of biosensors for early diagnosis and drug monitoring is also examined. Artificial intelligence (AI) is proposed as a supportive tool for dose predictions and 3D printing performance. Finally, future trends related to regulatory aspects for implementing 3D printing in clinical practice are addressed.
Download the full article as PDF here: 3D Printing of Dietary Products for the Management of Inborn Errors of Intermediary Metabolism in Pediatric Populations
or read it here
Carou-Senra, P.; Rodríguez-Pombo, L.; Monteagudo-Vilavedra, E.; Awad, A.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A.; Couce, M.L. 3D Printing of Dietary Products for the Management of Inborn Errors of Intermediary Metabolism in Pediatric Populations. Nutrients 2024, 16, 61. https://doi.org/10.3390/nu16010061
Read more on “3D Printing” here:


