Why 90% of clinical drug development fails and how to improve it?
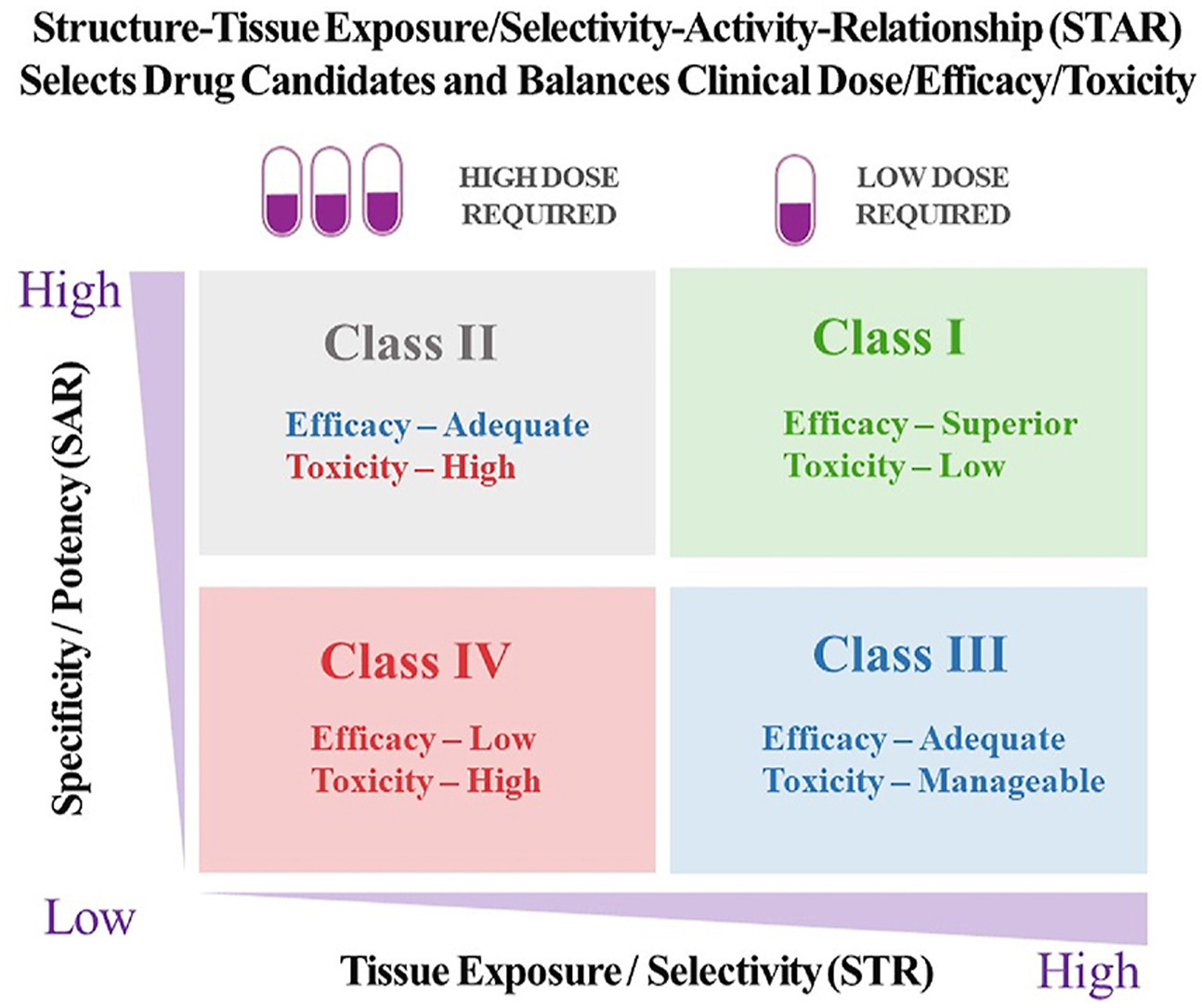
Ninety percent of clinical drug development fails despite implementation of many successful strategies, which raised the question whether certain aspects in target validation and drug optimization are overlooked? Current drug optimization overly emphasizes potency/specificity using structure‒activity-relationship (SAR) but overlooks tissue exposure/selectivity in disease/normal tissues using structure‒tissue exposure/selectivity–relationship (STR), which may mislead the drug candidate selection and impact the balance of clinical dose/efficacy/toxicity. We propose structure‒tissue exposure/selectivity–activity relationship (STAR) to improve drug optimization, which classifies drug candidates based on drug’s potency/selectivity, tissue exposure/selectivity, and required dose for balancing clinical efficacy/toxicity. Class I drugs have high specificity/potency and high tissue exposure/selectivity, which needs low dose to achieve superior clinical efficacy/safety with high success rate. Class II drugs have high specificity/potency and low tissue exposure/selectivity, which requires high dose to achieve clinical efficacy with high toxicity and needs to be cautiously evaluated. Class III drugs have relatively low (adequate) specificity/potency but high tissue exposure/selectivity, which requires low dose to achieve clinical efficacy with manageable toxicity but are often overlooked. Class IV drugs have low specificity/potency and low tissue exposure/selectivity, which achieves inadequate efficacy/safety, and should be terminated early. STAR may improve drug optimization and clinical studies for the success of clinical drug development.
Why 90% of clinical drug development fails?
Drug discovery and development is a long, costly, and high-risk process that takes over 10–15 years with an average cost of over $1–2 billion for each new drug to be approved for clinical use1. For any pharmaceutical company or academic institution, it is a big achievement to advance a drug candidate to phase I clinical trial after drug candidates are rigorously optimized at preclinical stage. However, nine out of ten drug candidates after they have entered clinical studies would fail during phase I, II, III clinical trials and drug approval. It is also worth noting that the 90% failure rate is for the drug candidates that are already advanced to phase I clinical trial, which does not include the drug candidates in the preclinical stages. If drug candidates in the preclinical stage are also counted, the failure rate of drug discovery/development is even higher than 90%.
Analyses of clinical trial data from 2010 to 2017 show four possible reasons attributed to the 90% clinical failures of drug development: lack of clinical efficacy (40%–50%), unmanageable toxicity (30%), poor drug-like properties (10%–15%), and lack of commercial needs and poor strategic planning (10%)2,4. Generally, drug development follows a classical process (Fig. 1), which includes vigorous genetic and genomic target validation, high-throughput screening (HTS) of drug candidate molecules, rigorously drug optimization for activity and drug-like properties, preclinical efficacy and toxicity testing, and biomarker-guided selection of patients and optimal clinical trial designs. In the past few decades, each step of the above drug development has been rigorously optimized and validated, while many successful strategies have been rightfully implemented in the drug development process to select the best drug candidates for clinical studies. Despite this validated effort, the overall success rate of clinical drug development remains low at 10%–15%. Such persistent high failure rate raises several questions: Why 90% of clinical drug development fails despite implementation of many successful strategies in the past several decades? Did we overlook certain aspects of drug development process leading to high failure? How can we improve the success rate of clinical drug development?
Continue reading here
About this article: Duxin Sun, Wei Gao, Hongxiang Hu, Simon Zhou, Why 90% of clinical drug development fails and how to improve it?Acta Pharmaceutica Sinica B, 2022, ISSN 2211-3835, https://doi.org/10.1016/j.apsb.2022.02.002.
(https://www.sciencedirect.com/science/article/pii/S2211383522000521)


