AEROSIL® 200 Pharma – well established and ready for tomorrow’s drug production
Continuous manufacturing is one of the greatest trends in the pharmaceutical industry, bringing advantages compared to the traditional batch production:
- Quality and cost position of the final medicine is improved
- Batch-to-batch variations are eliminated
- Intermediate storage becomes obsolete
- Potential future regulatory requirements are met.
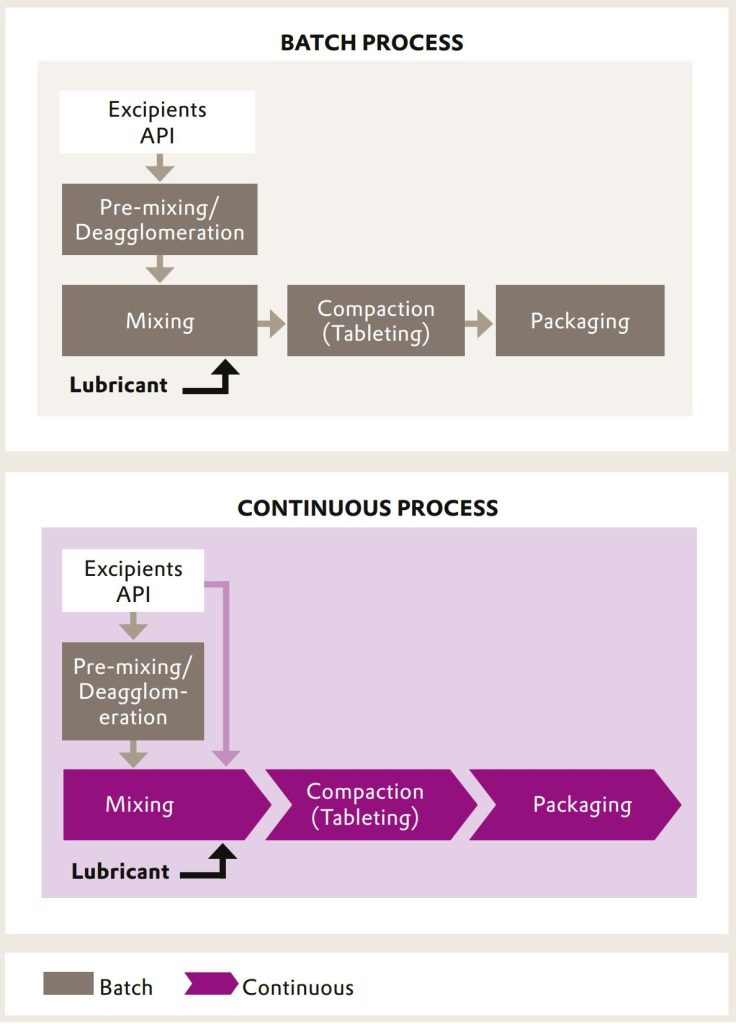
As part of continuous manufacturing, continuous direct compression requires the integration of continuous powder feeding units, a continuous dry powder mixer and tablet press. Together, these production steps build the core equipment for a successful implementation of this manufacturing technique of growing importance.
Besides the clear advantages, continuous direct compression creates some challenges for the manufacturing process. Exact dosing of small amounts of Active Pharmaceutical Ingredients (APIs) and excipients is key to ensure a properly working continuous process. There is no downtime during the continuous run. The process must operate with low maintenance. Any issues, for example in powder flow, can impair the continuous production.
for tomorrow’s drug production

The dosing of a glidant such as AEROSIL® 200 Pharma at very low feeding rates might be perceived as a challenge. With the proper feeding equipment, dosing of down to 100 g/h can be realized for the Evonik fumed silica.
The comparable low residence time of the materials in the continuous mixer has been shown to be sufficient to incorporate the AEROSIL® 200 Pharma into the system. The glidant is distributed on the surface of the host powder and can fulfill its function of decreasing the van der Waals forces, as well as, eliminating liquid bridges between particles in the mixture.
AEROSIL® Pharma is the industry standard glidant since decades. It ensures proper free flow within the tableting
equipment and a homogeneous distribution of APIs. In addition, tablet properties such as mass variation and
tablet hardness are improved by Evonik silica glidants. We at Evonik trust that continuous direct compression
needs a glidant to be implemented successfully. This was demonstrated in an internal case study in cooperation
with Gericke and L.B. Bohle.
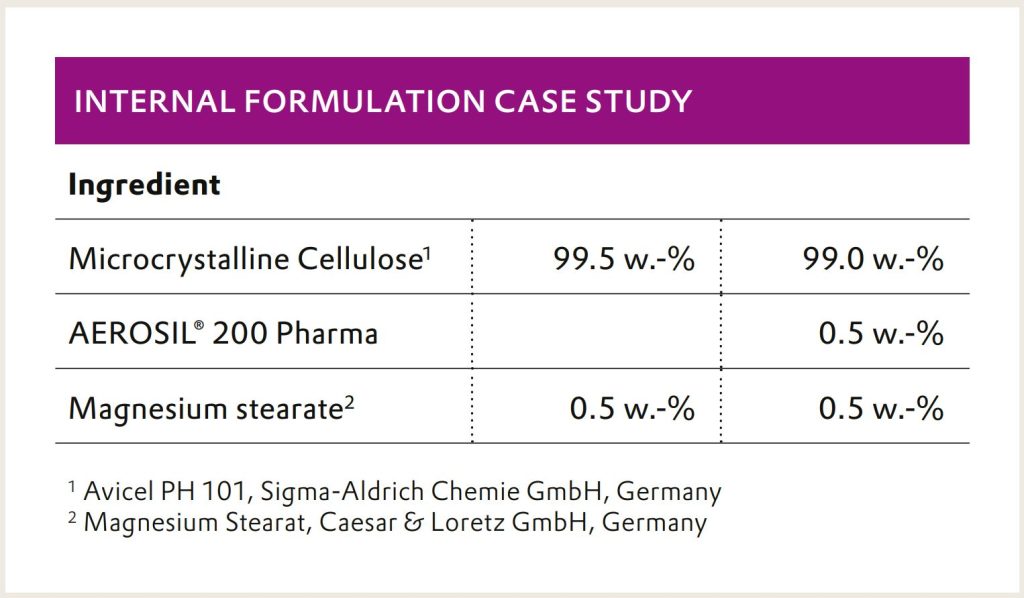
AEROSIL® 200 Pharma ensures the consistent powder
flow from the mixer to the tableting machine. It reduces
the retention of the powder in the tubing from the mixer
and facilitates the filling of the compression chamber.

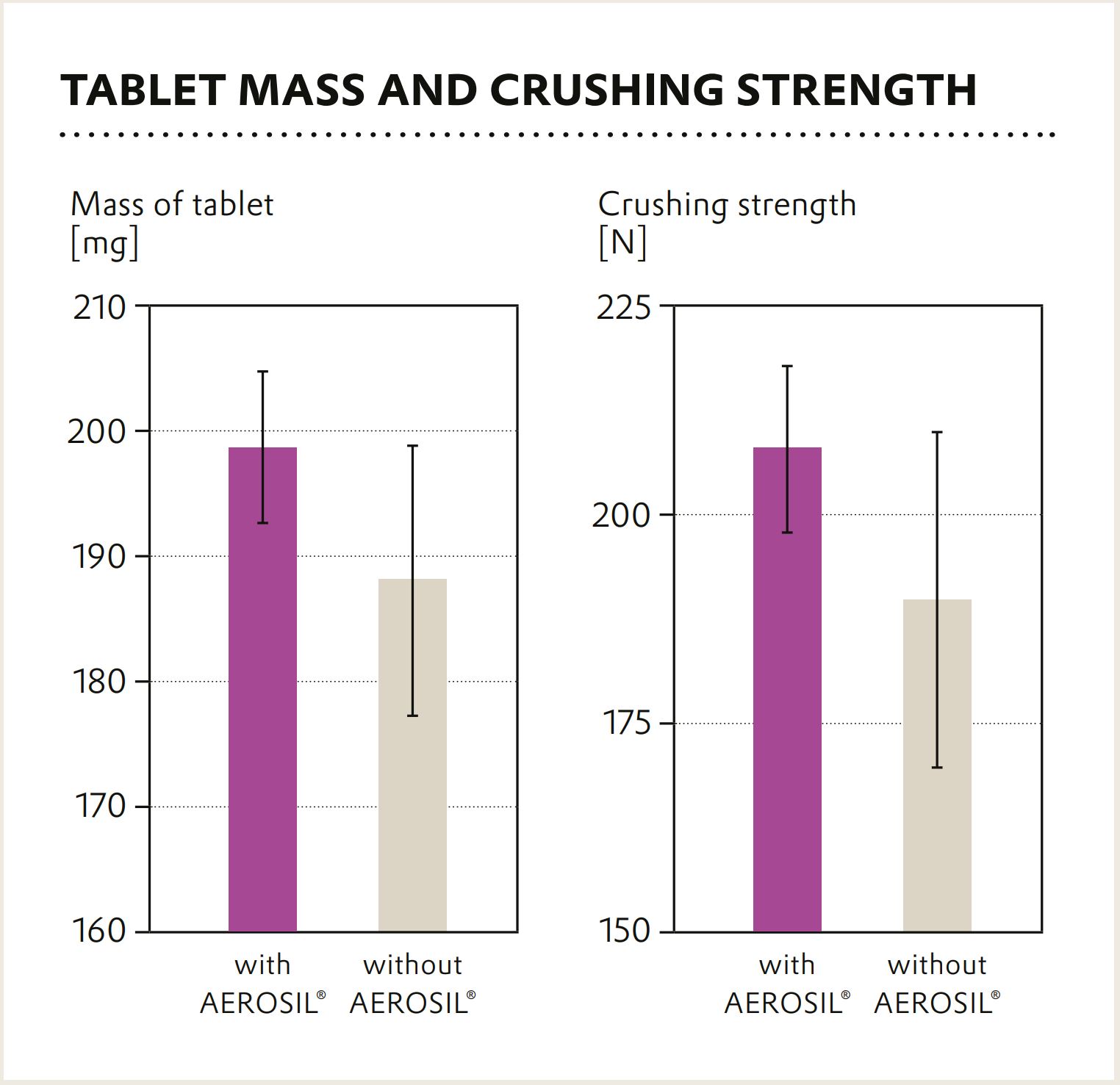
In the final tablets produced during the case study in the QbCon® System at L.B. Bohle, AEROSIL® 200 Pharma translates into higher tablet weights but strongly reduced tablet weight variation. In addition, the crushing strength is increased as well whereas its variance is decreased.
Overall, AEROSIL® 200 Pharma is not only suitable for continuous direct compression, it is a central excipient for homogeneous and stable tablets produced in the continuous process, now and in future technologies.
See the full technical information on “AEROSIL® 200 Pharma by Evonik” here
(click the picture to download the technical information)
Source: Evonik technical information “AEROSIL® 200 Pharma by Evonik“
Request a sample or more information here:


