AEROSIL® Pharma colloidal silicon dioxide – technical information

Evonik is one of the world’s leading CDMOs for advanced drug delivery. Our integrated portfolio of functional excipients, technologies and services for the formulation development, process scale-up and cGMP manufacturing of complex oral and parenteral drug products can reduce your project risk, boost performance and increase brand preference.
See the new technical information 1424 by Evonik of “AEROSIL® Pharma colloidal silicon dioxide” here:
AEROSIL®:
Designed for improved powder flow
and cost efficient solid dosage form production
1. Glidants: the fundament of cost efficient tablet production
Higher health care expenses due to the aging population constantly increase the cost pressure on pharmaceutical producers. The economy of production receives a stronger focus over the last few years, especially in generics manufacturing. Because of this, efficient processes like direct compression have seen a boost, since they require less processing steps and excipients in the formulation.
Successful implementation of direct compression tableting relies heavily on favorable flow conditions of the employed powder mixture1. Optimal powder flow is a prerequisite for highly productive tableting operations. On top of the economic factors, inadequate powder flow can lead to tablet weight variability issues and out of specification batches. AEROSIL® Pharma glidants2 help to optimize the fl ow of powder, which is vital for cost efficient production of high quality tablets.
Favorable powder flow is not only a prerequisite for direct compression but also relates to other processes in the health care industry, such as:
Filling of capsules and sachets with pharmaceutical powders
Filling capsules and powder sachets requires optimal powder flow to achieve high productivity and content uniformity.
Automatic dosage of ingredients
With the rise of continuous pharmaceutical production, good powder flow is key to have favorable productivity and consistency in production.
Handling of APIs with small crystal size
Crystal micronization can be an effective way to improve the dissolution of some APIs, as well as safeguarding the dosage uniformity of formulations containing highly potent actives. However, the smaller the API crystals get, flow becomes worse and the agglomeration tendency increases. Blending AEROSIL® Pharma colloidal silicon dioxide3 with these micronized APIs can inhibit the agglomeration, preserving the desired effect.
1.1. Theoretical models for powder flow
As favorable powder flow is a prerequisite for the production of solid dosage forms, it is important to understand the reasons why the flow of powders can be impeded. Typical models for the flow properties of powders look at the balance of forces that a particle experiences in a defined situation. Most models use the gravitational forces acting on the particle as the driver for powder flow. Attractive forces between the particles in the powder and between the particle and the container wall oppose the gravitational forces (see Figure 1).
Powders flow well if the gravitational forces are higher than the sum of the counteracting attractive forces. Typically, this is the case for powders with large particles and high particle density. For smaller and less dense particles, powder flowability deteriorates if the particle size falls below a critical value4.
The attractive forces can be due to van der Waals interactions, electrostatic (Coulomb) forces in dry powders, or liquid bridges between the particles if the powder is moist or hygroscopic. For dry powders at rest, with short inter-particle distances, van der Waals forces are the dominating attractions. Hamaker described these attractive forces for ideally spherical particles with the well-known equation given in Figure 2.
![]()
In moist or hygroscopic powders, liquids can gather in the dimples or pores of particles, and thereby result in the formation of inter-particle bridges. These bridges exert especially high attractive forces between the particles (figure 3).
2. Spotlight on AEROSIL® colloidal silicon dioxide glidants
AEROSIL® Pharma colloidal silicon dioxide is a preferred choice in pharmaceutical manufacturing due to its high purity, consistent quality and efficiency. Table 1 gives an overview of all AEROSIL® and AEROPERL® Pharma products with their characteristic physico-chemical properties and their compliance to the different pharmacopeia monographs.
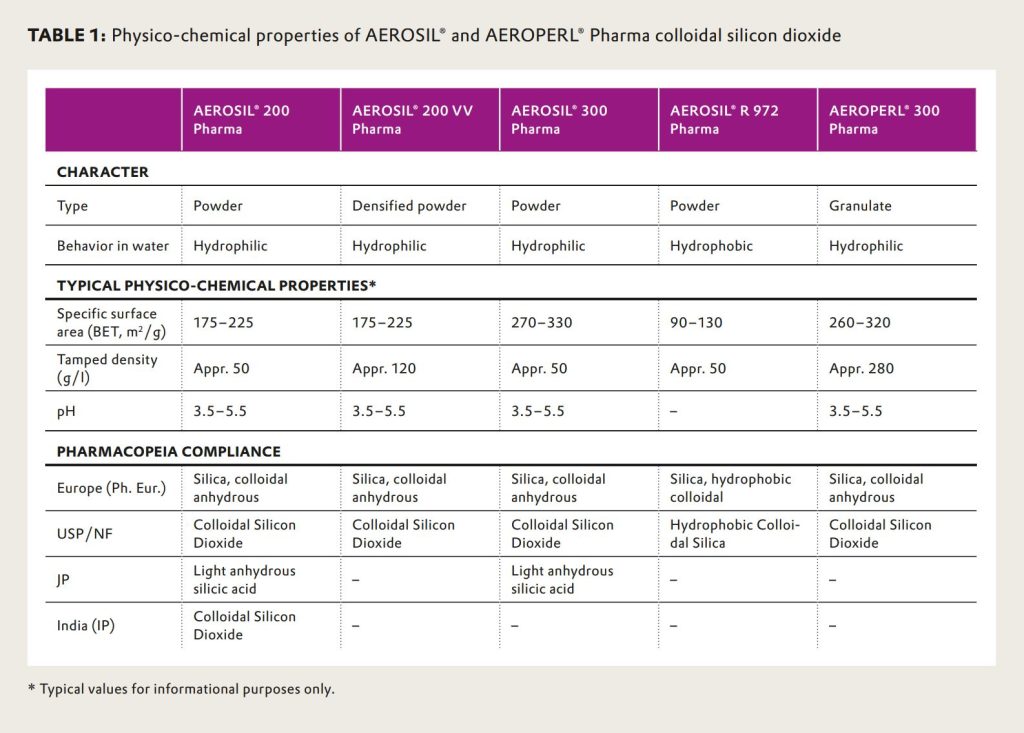
AEROSIL® 200 Pharma is the traditional glidant, helping to obtain the optimal powder fl ow required by today’s highspeed tablet presses. Since the fi rst time AEROSIL® 200 Pharma was used as a glidant9, challenges in formulating solid dosage forms have become more complex. Tablet powders of different average particle size, composition and moisture sensitivity are common in the industry, requiring specialized products to be able to compete in the increasingly cost sensitive health care environment. To support the industry to cope with these challenges, Evonik has broadened its AEROSIL® Pharma portfolio to provide optimal glidants for any tableting process.
AEROSIL® 200 VV Pharma is the glidant solution for customers facing limitations in their storage facility. This product is a densified version of AEROSIL® 200 Pharma that, on top of requiring less than half of the storage space, also creates less than half of the packing waste. The densification also leads to a less dusty product with stronger agglomerates, which makes it a preferred free flow additive for host powders with a higher particle size.
AEROSIL® 300 Pharma features an exceptionally high specific surface area. The material therefore has particularly favorable anti-caking performance. The addition of AEROSIL® 300 Pharma has a strong influence on tablet stabilities.
![]()
3. Powder flow enhancement with AEROSIL® Pharma colloidal silicon dioxide
CASE STUDY 1
Powder flow of microcrystalline cellulose and lactose of different particle size and their mixtures with AEROSIL® Pharma colloidal silicon dioxide products
FORMULATIONS, PROCESSING AND POWDER
FLOW ASSESSMENT
Simple binary powder mixtures of non-compression lactose and microcrystalline cellulose products with 0.5 w.-% AEROSIL® Pharma products were prepared. The host powders were selected to refl ect diff erent particle size ranges.
![]()
Equivalent volumes of the excipients were mixed with 0.5 w.-% of the different AEROSIL® Pharma products in a tumbling mixer (Turbula T2F, Willy Bachofen AG, Switzerland) for 10 min at a revolution of 67 rpm using equivalent fall heights of the powder in the mixing bottle.
The powder flow was assessed by the angle of repose method.
![]()
See the full technical information 1424 “AEROSIL® Pharma colloidal silicon dioxide” here
(click the picture to download the brochure)![]()
Source: Evonik technical information “AEROSIL® Pharma colloidal silicon dioxide”
See also the EVONIK Excipients Offering
Do you need more information or a sample of Aerosil® or other excipients by Evonik?

