Buccal delivery system of active pharmaceutical ingredients-ionic liquid (API-IL): Effects of API-IL loading and gelatin film concentration
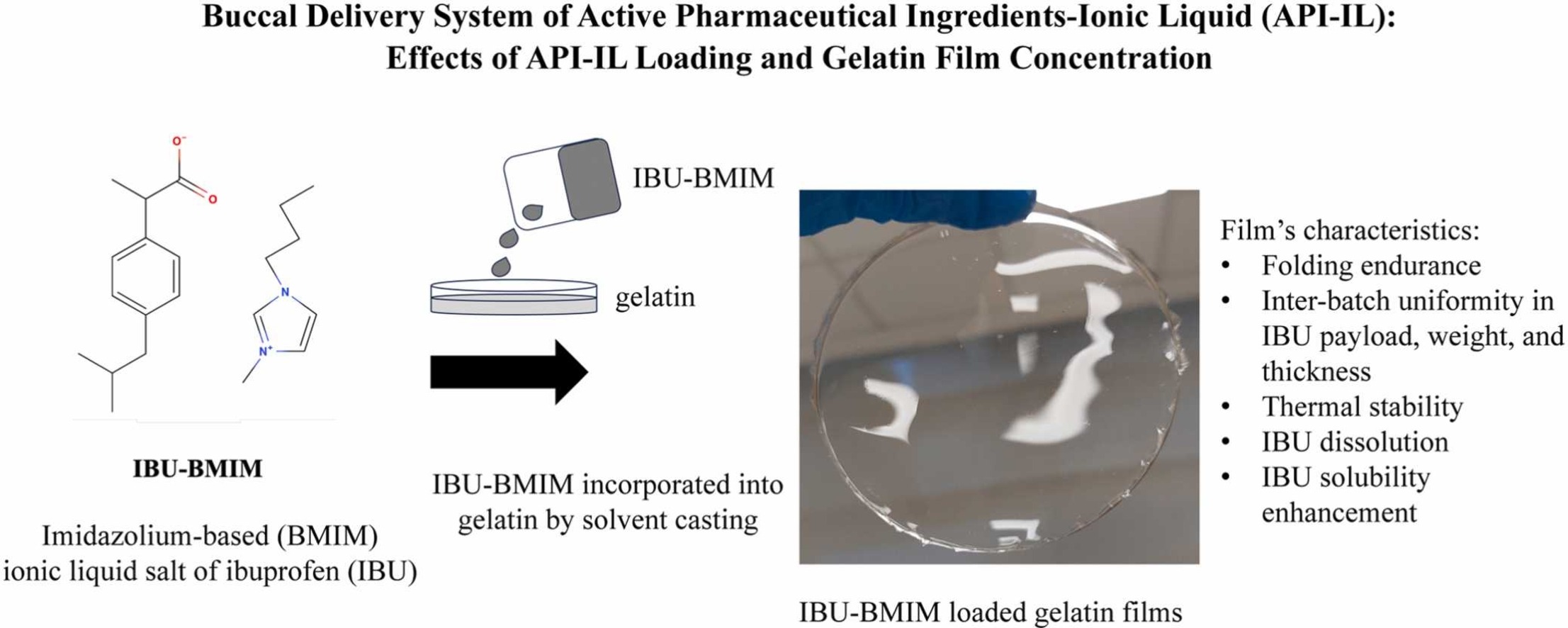
Ionic liquid (IL) salt of active pharmaceutical ingredient (API) represents a promising formulation strategy to address low drug solubility and polymorphism prevalent in API solid crystals. The present work developed for the first time a buccal delivery system of API-IL via fast-dissolving API-IL-loaded gelatin films. Imidazolium-based ibuprofen salt was used as the model API-IL. The effects of API-IL loading and gelatin concentration on the film’s (i) mechanical strength, (ii) inter-batch uniformity in the films’ API payload, weight, and thickness, (iii) thermal stability, (iv) API dissolution and solubility enhancement were investigated. The plasticizer role of API-IL was evident, where minimum 30 wt% API-IL loading was needed to produce flexible yet mechanically-strong films. Lower API-IL loading produced brittle films due to insufficient plasticization facilitated by hydrogen bond interactions between API-IL and gelatin. Gelatin concentration influenced films’ mechanical strength, weight/thickness, and API dissolution rate. Depending on the API-IL loading and gelatin concentration, films with API payload (7–30 mg/cm2), thickness (300–900 µm), and weight (20–110 mg/cm2) were produced at nearly 100% efficiency and high inter-batch uniformity. API-IL existed as amorphous liquid in the film exhibiting fast API dissolution (100% in 15 min) and high kinetic solubility (8 times thermodynamic solubility) in simulated saliva fluid.
Introduction
Approximately 70% to 80% of newly discovered active pharmaceutical ingredients (APIs), as well as 40% of commercial APIs, are categorized as poorly water-soluble compounds, resulting in their low bioavailability after administration (Di et al., 2012). Various drug solubility enhancement strategies, such as co-crystallization, size comminution, amorphous solid dispersion, micellization, salt formation, hydrate and solvate formation, and prodrug approach have been developed (Khadka et al., 2014, Lim et al., 2018). Besides the poor aqueous solubility, another prevalent issue plaguing solid forms of the API is polymorphic conversions, where API crystals undergo structural transformations during manufacturing or storage into different crystal forms, which exhibit distinct physicochemical characteristics from the intended crystal form (Higashi et al., 2017).
One promising strategy to simultaneously solve the low solubility and polymorphism issues is by converting the API into its ionic liquid salt form known as API ionic liquid (or in short API-IL) (Wu et al., 2021). API-ILs are made up of stoichiometric blends of completely ionized API molecules and the counterions typically characterized by melting points below 100 °C (Balk et al., 2015). In fact, most API-ILs exist as liquid at room temperature caused by the bulky asymmetric cations and weakly fitting anions that lead to destruction of the crystal lattice (Egorova et al., 2017). Compared to API crystals, API-ILs exhibit superior aqueous solubility and fast dissolution owed to their lack of crystalline structure (Balk et al., 2015). By virtue of their liquid form, API-ILs do not have the issue of polymorphism prevalent in API crystals (Wu et al., 2021). Nevertheless, most API-ILs exist as viscous liquids that are highly hygroscopic, hence bringing forth challenges in their handling, dosing precision, and storage stability (Ng et al., 2022). Consequently, delivery of API-ILs has been limited to the transdermal delivery route in the form of microemulsion (Lu et al., 2022).
To facilitate oral delivery of API-ILs, which is the preferred drug administration route by patients (Sohail Arshad et al., 2021), API-ILs must be formulated into particulate carriers to improve their handling and dosing accuracy, and importantly protect them from the surrounding humidity. Examples of particulate carriers that have been studied include polysaccharides (Stocker et al., 2020, Tsolaki et al., 2021), proteins (Chaunier et al., 2020), and silica (Bica et al., 2012, Viau et al., 2010). Besides the oral route, the buccal route represents a highly feasible administration route for API-ILs, particularly for APIs that are prone to first-pass metabolism (Jacob et al., 2021). Buccal administration can effectively deliver drugs for both local and systemic effects, via drug absorbance through the mucosal membrane lining the cheek. The buccal cavity is extensively vascularized with much less enzymatic activity than the gut (Barua et al., 2016). The buccal route enables drugs to steer clear of acid hydrolysis in the stomach and bypass the hepatic first-pass effect, resulting in enhanced bioavailability and a lower dosage requirement to achieve the same therapeutic effects (Jacob et al., 2021). The buccal route also enables quick removal of the drug if adverse effects are observed (e.g., drug allergy).
To the best of our knowledge, buccal delivery systems of API-ILs have rarely been investigated. In fact, there were very few studies on buccal drug delivery involving ILs. A study by (Vaidya and Mitragotri, 2020) explored the addition of IL made up of choline and geranic acid into a thin polymer film aimed at improving insulin permeation across the buccal membrane. Recently, (Tagami et al., 2022) used 3D printing to prepare buccal films made up of Eudragit and hypromellose polymers containing API-IL (i.e., ibuprofen-lidocaine). Their study focused on the feasibility of employing the 3D printing technique to prepare buccal films containing API-IL. Key quality attributes (e.g., folding endurance, thickness, drug content uniformity) of the buccal films were not characterized. Solubility enhancement capability of the API-IL embedded in the film was not examined either.
In the present work, we developed buccal film formulation of API-IL using the well-established, simple, and economical solvent casting method. Imidazolium-based IL of a poorly soluble drug ibuprofen (IBU) was used as the model API-IL. The ibuprofen-imidazolium has been demonstrated in several studies to exhibit similar cytotoxicity as the native IBU towards various human cell lines, including ovarian, intestinal, liver, and skin cells (Bastos et al., 2022, Santos et al., 2019, Wu et al., 2019). In fact, the similar cytotoxicity of imidazolium-based API-IL compared to the native API has also been demonstrated for other APIs, for example, ampicillin (Ferraz et al., 2015).
Gelatin was used as the matrix former for the buccal films owed to gelatin’s good film forming ability, biocompatibility as recognized by its Generally Recognized as Safe (GRAS) status, mechanical rigidity, and mucoadhesive properties (Jovanović et al., 2021). Moreover, incorporations of ILs into a gelatin matrix for other applications (e.g., antimicrobial food packaging (Mehta and Kumar, 2019), biosensors (Sharma et al., 2015, Singh et al., 2017), medicated patches (Maneewattanapinyo et al., 2019)) have been demonstrated not to cause any adverse effects on the ILs (e.g., dissociation).
As the plasticizing effect of ILs on macromolecules have been widely reported (Bendaoud and Chalamet, 2014, Ren et al., 2020), we prepared the gelatin buccal films without using a plasticizer, thereby simplifying the films’ formulation to only two variables, namely (1) API-IL loading and (2) gelatin concentration. We investigated the effects of API-IL loading and gelatin concentration on the feasibility of forming thin gelatin films with good mechanical strength as characterized by their folding endurance. Molecular interactions between gelatin and API-IL were characterized by infrared spectroscopy and their impacts on the physical form of API-IL in the gelatin film were examined by X-ray diffraction and thermal analysis. For the feasible formulations (i.e., those with sufficient folding endurance), the films’ (i) API payload, (ii) drug entrapment efficiency, (iii) inter-batch uniformity of the API payload, (iv) weight and thickness uniformity, (v) thermal stability, (vi) API dissolution rate, and (vii) API solubility enhancement in the simulated saliva fluid, were characterized.
Materials
Ibuprofen sodium salt (IBU-Na), 1-butyl-3-methylimidazolium chloride (BMIMCl), gelatin (Product number: 48723, tested according to Ph. Eur), trifluoroacetic acid, disodium phosphate (Na2HPO4.7H2O), potassium dihydrogen phosphate (KH2PO4), sodium chloride (NaCl), and phosphoric acid (H3PO4) were purchased from Sigma Aldrich (Singapore). Chloroform-D (CDCl3, 99.8%) was purchased from Cambridge Isotope Laboratories (USA). Ethanol (≥ 99%) and acetone (≥ 99.5%) were purchased from Aik Moh Paints.
Read more
Liu Han Ng, Kunn Hadinoto, Buccal delivery system of active pharmaceutical ingredients-ionic liquid (API-IL): Effects of API-IL loading and gelatin film concentration, Chemical Engineering Research and Design, Volume 202, 2024, Pages 115-125, ISSN 0263-8762,
https://doi.org/10.1016/j.cherd.2023.12.027.

