Nanostructured lipid carriers loaded into in situ gels for breast cancer local treatment
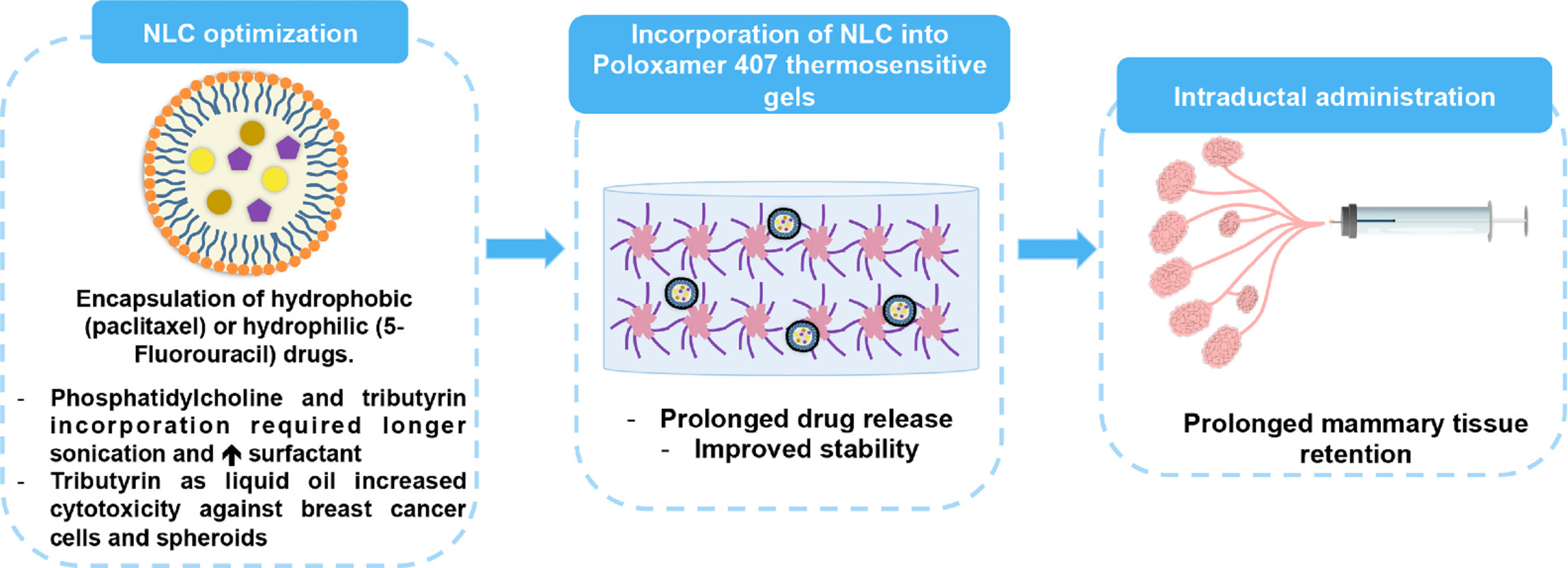
In this study, nanostructured lipid carriers (NLC) were developed and employed to obtain in situ thermosensitive formulations for the ductal administration and prolonged retention of drugs as a new strategy for breast cancer local treatment. NLC size was influenced by the type and concentration of the oil phase, surfactants, and drug incorporation, ranging from 221.6 to 467.5 nm. The type of liquid lipid influenced paclitaxel and 5-fluorouracil cytotoxicity, with tributyrin-containing NLC reducing IC50 values by 2.0-7.0-fold compared to tricaprylin NLC in MCF-7, T-47D and MDA-MB-231 cells. In spheroids, the NLCs reduced IC50 compared to either drug solution (3.2–6.2-fold). Although a significant reduction (1.26 points, p<0.001) on the health index of Galleria mellonella larvae was observed 5 days after NLC administration, survival was not significantly reduced.
To produce thermosensitive gels, the NLCs were incorporated in a poloxamer (11%, w/w) dispersion, which gained viscosity (2-fold) at 37°C. After 24 h, ∼53% of paclitaxel and 83% of 5-fluorouracil were released from the NLC; incorporation in the poloxamer gel further prolonged release. Intraductal administration of NLC-loaded gel increased the permanence of hydrophilic (2.2-3.0-fold) and lipophilic (2.1-2.3-fold) fluorescent markers in the mammary tissue compared to the NLC (as dispersion) and the markers solutions. In conclusion, these results contribute to improving our understanding of nanocarrier design with increased cytotoxicity and prolonged retention for the intraductal route. Tributyrin incorporation increased the cytotoxicity of paclitaxel and 5-fluorouracil in monolayer and spheroids, while NLC incorporation in thermosensitive gels prolonged tissue retention of both hydrophilic and hydrophobic compounds.
Download the full article as PDF here Nanostructured lipid carriers loaded into in situ gels for breast cancer local treatment
or read it here
Materials
Polysorbate 80 (Tween 80), sorbitan oleate (Span 80), tributyrin, decyl glucoside and poloxamer 407 (Pluronic 127) were obtained from Sigma (St. Louis, MO, USA). Paclitaxel was purchased from Cayman Chemical (Ann Arbor, MI, USA) and soy phosphatidylcholine (PC) and 1-palmitoyl-2-(6-((7-nitro-2-1,3-benzoxadiazol-4-yl)amino)hexanoyl)-sn-glycero-3-phosphocholine (NBD-PC) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). 5-Fluorouracil were obtained from Oakwood Chemical (Estill, SC, USA). Rhodamine B and propylene glycol were acquired from Synth (São Paulo, SP, Brazil) and Alexa Fluor 647 was obtained from ThermoFisher Scientific (Waltham, MA, USA). Tricaprylin was kindly supplied by Croda Health Care (Edison, NJ, USA), and glyceryl behenate (Compritol® 888 ATO) by Gattefosse (Saint-Priest, France). Ultrapure water was used unless stated in the individual methods.
Julia S. Passos, Alexsandra C. Apolinario, Kelly Ishida, Tereza S. Martins, Luciana B. Lopes, Nanostructured lipid carriers loaded into in situ gels for breast cancer local treatment, European Journal of Pharmaceutical Sciences, 2023,
106638, ISSN 0928-0987, https://doi.org/10.1016/j.ejps.2023.106638.
Read also more on Excipients for Parenterals here:


