Impact of unloading kinematics on the occurrence of capping during the production of pharmaceutical tablets
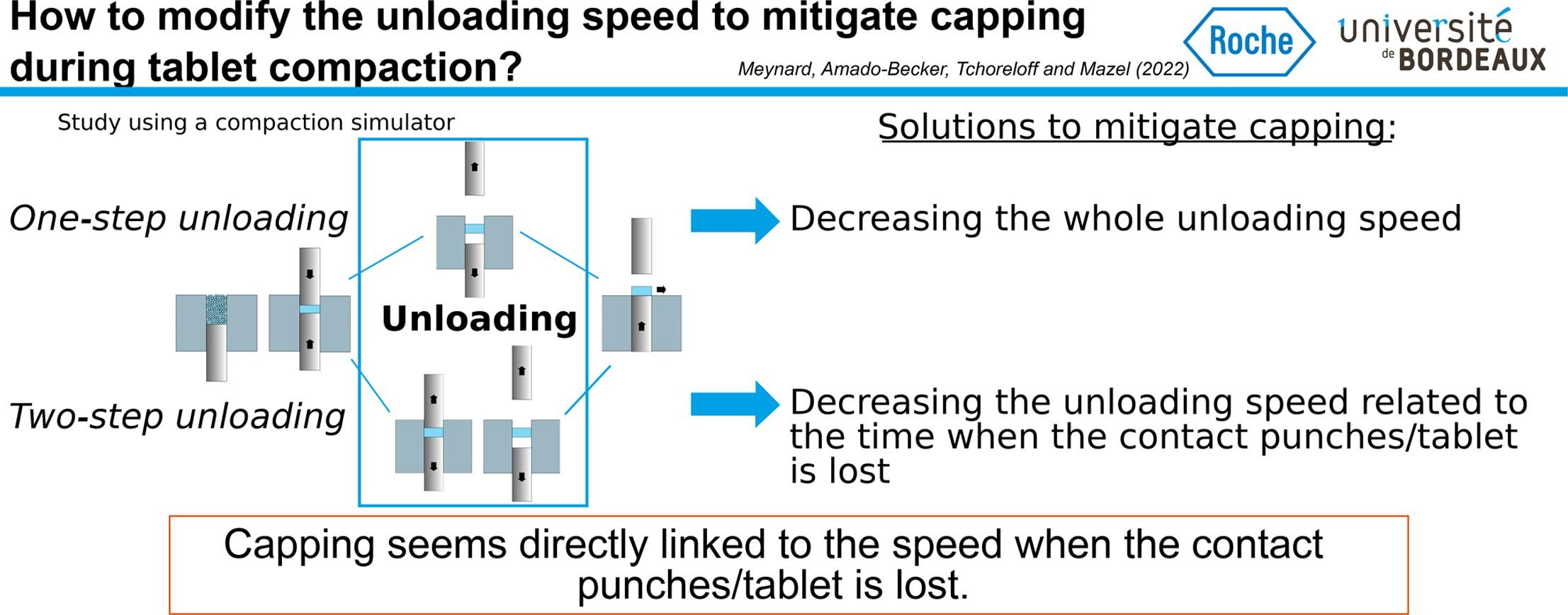
Capping is a common defect that can occur during the manufacturing of pharmaceutical tablets. Several studies showed that decreasing the unloading speed of the manufacturing cycle plays a role in the occurrence of such defects. Following this idea, we study in this work the influence of the unloading step on capping using a compaction simulator.
Measuring the die wall pressure made it possible to detect precisely that tablets capped just after the unloading (some milliseconds only). To evaluate the impact of the unloading speed on capping, we developed a two-step unloading phase controlled by three manufacturing parameters. It was possible to mitigate capping by decreasing the speed at which the contact between the punches and the tablet was lost. Capping seemed due to dynamical effects related to the release of the axial pressure. The modification of the unloading step to mitigate capping led to significant changes in tablet density but no clear trends were found for the residual die-wall pressure and tablet strength.
This work made it possible to improve the understanding of capping. Moreover, the two-step unloading cycle gave a new idea for possible modifications that could be done on rotary presses in order to mitigate capping.
Continue reading here
About this article: J. Meynard, F. Amado-Becker, P. Tchoreloff, V. Mazel, Impact of unloading kinematics on the occurrence of capping during the production of pharmaceutical tablets, International Journal of Pharmaceutics, Volume 621, 2022, 121818, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2022.121818. (https://www.sciencedirect.com/science/article/pii/S0378517322003738)
Raw materials
The following products were used for this study:
- calcium sulfate dihydrate “Comp” (Compactrol, JRS Pharma, Rosenberg, Baden-Wurtemberg, Germany),
- mannitol “Man” (Mannitol, Cooper, Melun, Seine-et-Marne, France),
- microcrystalline cellulose “MCC” (Vivapur 12, JRS Pharma, Rosenberg, Baden-Wurtemberg, Germany),
- granulated lactose “MLac” (SuperTab 30GR, DFE Pharma, Goch, Nordrhein-Westfalen, Germany),
- crosscaramellose sodium “CCNa” (Vivasol, JRS Pharma, Rosenberg, Baden-Wurtemberg, Germany),
- magnesium stearate “MgSt” (Ligamed MF-2-V, Peter Greven, Bad Münstereifel, Nordrhein-Westfalen, Germany).
10 Errors that can occur during the coating process and how to avoid them
Learn more about errors that can occur during the coating process


