Feasibility study of the use of a homemade direct powder extrusion printer to manufacture printed tablets with an immediate release of a BCS II molecule
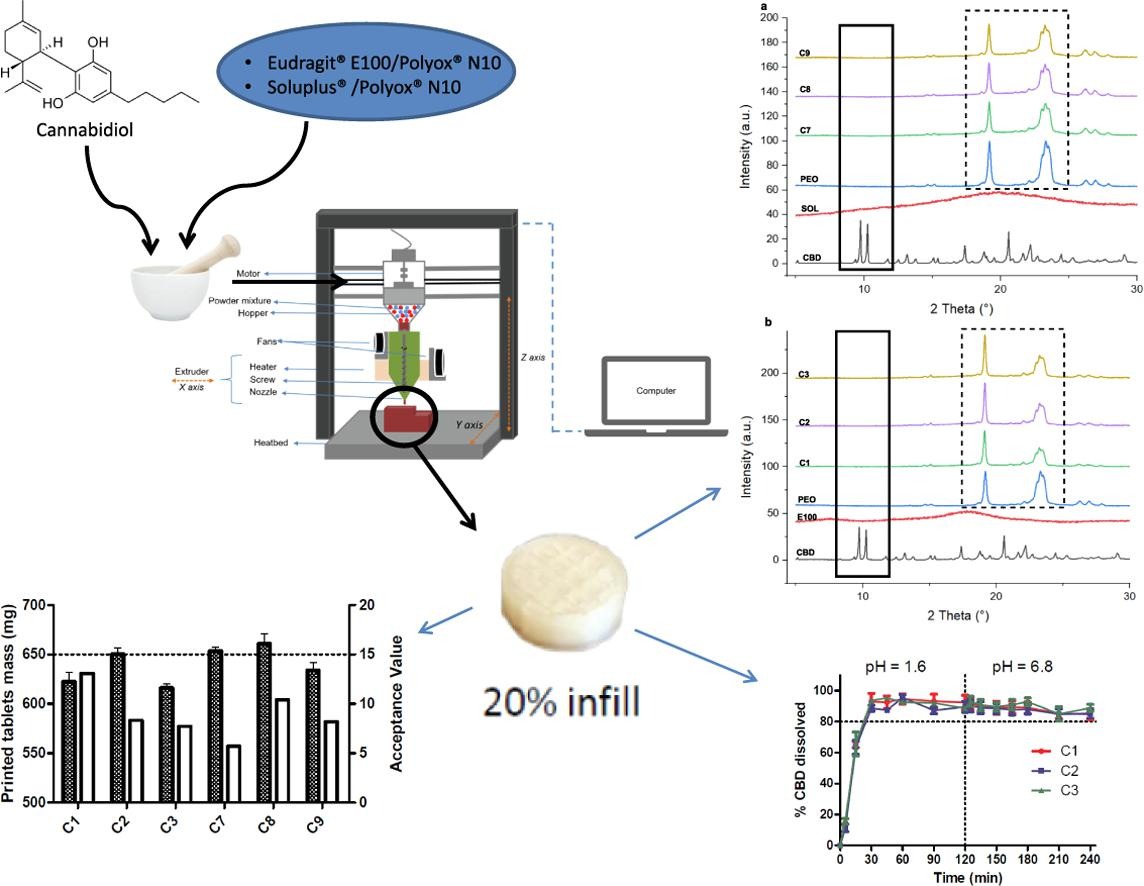
Among the various 3D printing techniques, FDM is the most studied in pharmaceutical research. However, it requires the fabrication of filaments with suitable mechanical properties using HME, which can be laborious and time-consuming. DPE has emerged as a single-step printing technique that can overcome FDM limits as it enables the direct printing of powder blends without the need of filaments. This study demonstrated the manufacturing of cylindrical-shaped printed tablets containing CBD, a BCS II molecule, with an immediate release. Different blends of PEO/E100 and PEO/SOL, each with 10 % of CBD, were printed and tested according to the Eur. Ph. for uncoated tablets. Each printed cylinder met the Eur. Ph. specifications for friability, mass variation and mass uniformity. However, only the E100-based formulations enabled a CBD immediate release, as formulations containing SOL formed a gel once in contact with the dissolution medium, reducing the drug dissolution rate.
Introduction
3D printing is an additive manufacturing technique able to convert a computer-aided design model into a physical object by a layer by layer deposition of material (Omari et al., 2022). This technique differs from traditional drug product manufacturing techniques in many ways. It allows to manufacture complex geometries (Lamichhane et al., 2019), different dosage forms (Jiang et al., 2019, Trenfield et al., 2019), drug products combining several incompatible active pharmaceutical ingredients (APIs) (Gioumouxouzis et al., 2018), complex drug delivery devices (Dumpa et al., 2021, Beg et al., 2020) and also original and unique shapes that increase patient compliance (Vaz and Kumar, 2021).
Among the various 3D printing techniques, fused-deposition modeling (FDM) is the most studied in pharmaceutical research due to its time-saving and cheap process, small size and accuracy (Rahim et al., 2019). FDM process consists of the extrusion of a filament made of thermoplastic polymers and one or several APIs and the deposition layer by layer of the molten material until the final printed shape is obtained. It allows to manufacture solid dosage forms with tailored doses (Crișan et al., 2022), release (Shi et al., 2021) or shape (Goyanes et al., 2015) or different drugs (Pereira et al., 2019), which proves its usefulness for personalized medicine (Khalid and Billa, 2022, Sandler et al., 2016). However, this technique has some drawbacks. Prior to printing, the filament must generally be prepared by hot-melt extrusion (HME) that involves temperatures above Tg of polymers, which is not suitable for thermosensitive APIs. Moreover, filaments for FDM printing are challenging to obtain. Indeed, they must have specific mechanical properties, i.e., suitable brittleness and flexibility. In most cases, a certain amount of plasticizer has to be added to the formulation to adjust filament flexibility, which is not desired for the physical state stability of the drug. The filament diameter is another parameter to control. It must be homogeneous and can vary between 1.75 and 3.00 mm depending on the printer model used (Jennotte et al., 2020). Filament diameter control often requires the use of expensive additional equipment, especially a conveyor belt, but also a melt pump or a filament maker (Parulski et al., 2021). Another method for filament loading is the immersion of a filament in a solution that contains the drug which penetrates into the filament by passive diffusion. This immersion method is suitable for thermosensitive drugs, but the drug loading is limited, generally to maximum 3 % (Fernández-García et al., 2020).
Direct powder extrusion 3D printing (DPE) may be useful to circumvent FDM drawbacks. This technique was first developed for the plastics industry (Liu et al., 2017). It was recently implemented in the pharmaceutical field by Goyanes and his team who successfully manufactured itraconazole printed tablets with different grades of HPC (Goyanes et al., 2019). The process implies the feeding of a powder mixture into the printer hopper, heating and extrusion followed by the deposition, layer by layer of the molten material according to the previously digitally designed geometry. This one-step direct printing of powder avoids the need of filaments manufacture by HME which significantly reduces process time and waste. The thermal stress underwent by the API is also reduced since the DPE induces only one heating step, while there are two during FDM process, making DPE as a good alternative for the printing of thermosensitive drugs. Additionally, DPE can overcome the limitations encountered in HME regarding high drug loading. Indeed, the formulation of high drug loaded filaments usually requires additional excipients in order to obtain not too-brittle or too-flexible filament, which is not the necessary for DPE (Sánchez-Guirales et al., 2021). This technique has already proven its potential to print objects with various applications in the pharmaceutical field (Wang et al., 2023, Ong et al., 2020, Maurizii et al., 2023, Moroni et al., 2022). For example, Goyanes et al. used different grades of hydroxypropylcellulose to manufacture printed tablets with a sustained release of itraconazole (Goyanes et al., 2019). In another study, the ability of DPE to manufacture mini printed tablets with a high drug loading (25 %) was demonstrated. The small size on the mini printed tablet allowed it to fit in a zero-size capsule in order to be combined with other mini printed tablets, oh other API, for treatment of disease requiring polypharmacy within a single dosage form (Sánchez-Guirales et al., 2021). Later, Malebari et al. investigated DPE for the printing of a combination of lopinavir/ritonavir in a pediatric dosage with HPMC and polyethylene glycol (PEG) in order to circumvent drug precipitation problem at intestinal pH observed with the administration of marketed drug Kaletra® (Malebari et al., 2022). Printed tablets were characterized by a zero-order sustained release drug during in vitro dissolution tests, which is promising for treating children with HIV. DPE also offers the possibility to print blends containing cyclodextrins as reported by Pistone et al. (2022). Indeed, authors successfully printed mixtures composed by niclosamide, hydroxypropyl-β-cyclodextrin (HPβCD) and PEG 6000. After in vitro dissolution tests, the solubility increase ability of HPβCD for poorly soluble drugs was highlighted.
Immediate release dosage forms, defined by the European Pharmacopoeia (edition 11.2, monograph 5.17.1) as forms allowing a drug dissolution of 80 % within 45 min, were also printed using DPE, although the studied drugs belonged to BCS class I, having a high aqueous solubility (Fanous et al., 2020, Mendibil et al., 2021, FDA, 2018). To the best of our knowledge, no BCS II drugs with an immediate release has been successfully printed by DPE.
Therefore, the aim of this work was to print solid dosage forms allowing an immediate release of a BCS II drug, namely cannabidiol (CBD). This drug was chosen as a BCS II model drug for two reasons. Firstly, because this molecule has a low oral bioavailability, partly due to its low aqueous solubility (0.1 µg/mL in water) (Koch et al., 2020). Secondly, CBD is being studied for many therapeutic properties, such as opioids use disorder, social anxiety, schizophrenia or cancers with a wide range of dosages varying from less than 1 mg/kg/day to 50 mg/kg/day (Bergamaschi et al., 2011, Heider et al., 2022, McGuire et al., 2018). The majority of clinical studies for this promising drug are performed with liquid formulations, probably because it is the fastest and least expensive way to perform CBD dosage titrations. Indeed, conventional manufacturing methods such as tableting often hinder the rapid progress through pre-clinical studies, due to being dose inflexible, high costs and causing high waste (Millar et al., 2019, Seoane-Viaño et al., 2021). DPE process could reduce time, cost and waste of these trials, given that it is sufficient to feed the exact amount of powder mixture with a specific drug loading needed for the study. Moreover, formulations printed by DPE are solid forms, generally better accepted by the patients in comparison to liquid formulations (Vasconcelos et al., 2007, Morishita and Peppas, 2006).
A powder-based homemade printer was used to produce the printed tablets. CBD was previously mixed with Eudragit® E100 (E100) or Soluplus® (SOL), known for their ability to increase solubility of poorly soluble drugs, and Polyox® N10 (PEO) as a plasticizer, before being printed. The obtained printed tablets were evaluated in terms of immediate release and tested according to the European Pharmacopeia monograph for uncoated tablets, as to this day there is no official guidelines regarding the evaluation of the performances or the quality control of a final 3D printed product yet (Mohapatra et al., 2022).
This study represents the first attempt to produce printed tablets containing a BCS II molecule with an immediate release, using a DPE process. It is expected to prove the interest and the robustness of this new 3D-printing technique for on-demand formulations. In the context of actual medicines shortages, DPE could provide a point-of-care rapid solution.
Materials
CBD was purchased from THC Pharm (Frankfurt, Germany). E100 (amino alkyl methacrylate copolymer, Tg: 50 °C, soluble in water at pH less than 5, gifted by Evonik, Germany) were used as matrix former. PEO (MW 100,000, gifted by Colorcon, UK), a semi-crystalline polymer (Tg = −67 °C and Tm = 65–70 °C) was used for its plasticizing effect. SOL (Polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer, Tg = 67 °C) was kindly received from BASF Chemical Co. (Ludwigshafen, Germany).
Read more
O. Jennotte, N. Koch, A. Lechanteur, F. Rosoux, C. Emmerechts, E. Beeckman, Brigitte Evrard, Feasibility study of the use of a homemade direct powder extrusion printer to manufacture printed tablets with an immediate release of a BCS II molecule, International Journal of Pharmaceutics, Volume 646, 2023, 123506, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2023.123506.
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


