Preparation and formula optimization of cephalexin loaded transferosomal gel by QbD to enhance the transdermal delivery: In vitro, ex vivo and in vivo study
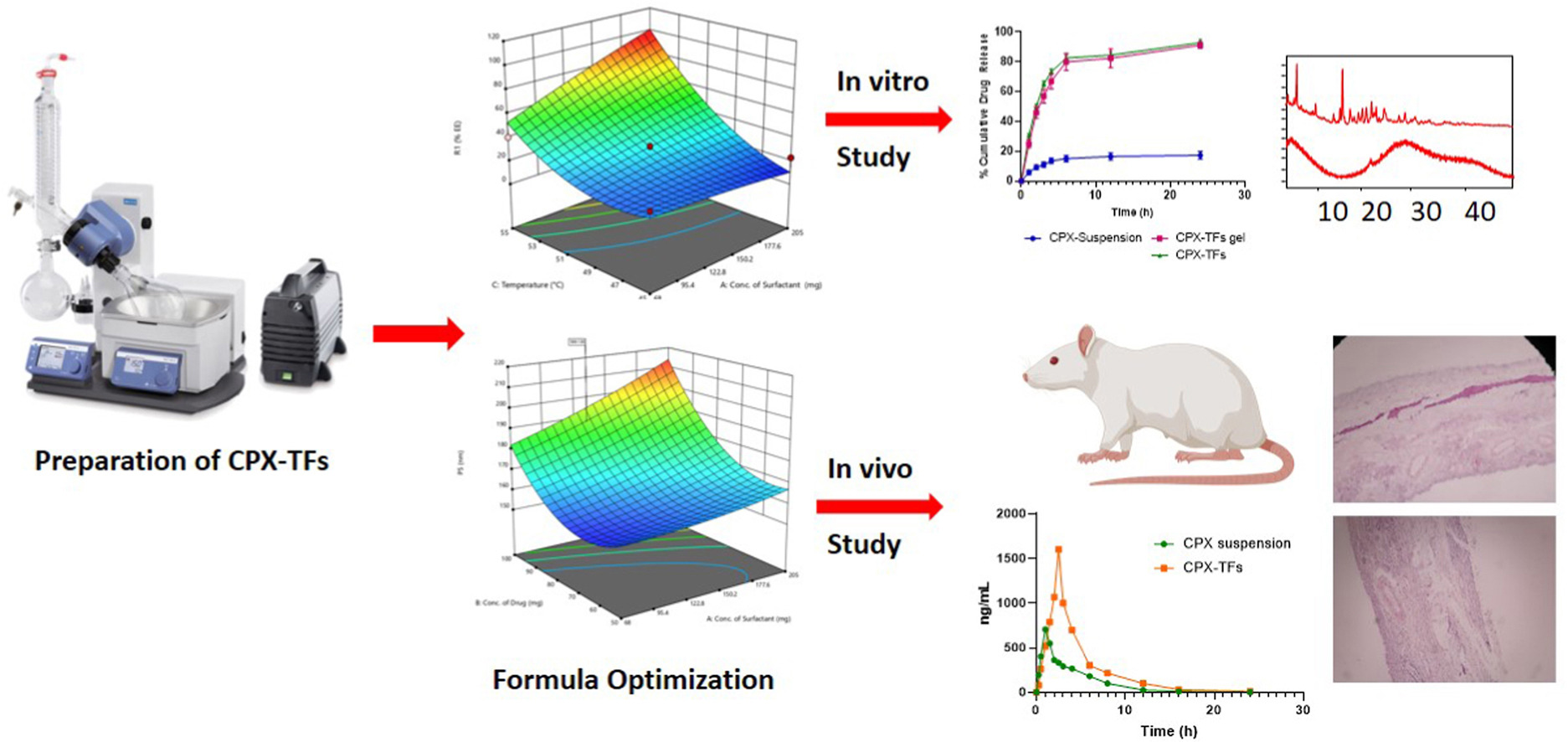
Most antibiotics used in topical formulations are not absorbed by deeper skin layers and subdermal tissues. Hence the skin infections are treated with large doses of oral or parenteral antibiotics which lead to unpleasant side effects with drug resistance. This could be the necessity for the preparation of transdermal drug delivery systems, which addressed the issues with administration. The present study designed to develop cephalexin loaded transferosomal (CPX-TFs) gel for an effective treatment of skin infection by improving transdermal delivery. The transferosomes (TFs) were prepared by thin film hydration method by employing QbD based Box-Behnken design. The major objectives of the study was to prepare CPX-TFs with higher entrapment and lower particle size, to enhance the penetration deeper into the skin layers. The seventeen different batches considering concentrations of phospholipon® 90H, sodium deoxycholate at different temperature condition were prepared and the formula was optimized. These variables showed positive effect on its entrapment efficiency and on particle size of transferosomes. The TFs with lower vesicle size showing better stability whereas in vitro diffusion study demonstrated cumulative release (92.96 ± 2%) up to 24 h. The optimized CPX-TFs were incorporated in carbopol 934 gel (0.25%, 0.5%, 1%, 1.5%, and 2% w/v) by dispersion method. This gel with 96.52 ± 3% (2.5% w/v) drug content demonstrated about 91.26 ± 2% release of CPX over a period of 24 h, showing biphasic drug release pattern (initial burst release in first 6 h then sustaining the release over 24 h). It shows excellent anti-bacterial action against the E coli, S aureus and K pneumonaie. The optimized CPX‒TFs gel presented maximum flux value than pure CPX suspension. The increased in permeation with gradual increased in surfactant concentration was observed. The in vivo pharmacokinetic study shows steady state plasma concentration and Cmax for CPX-TFs gel was found to be 2.3-fold which was comparatively greater than oral CPX suspension. The histopathological study confirms the safety of transdermal application of CPX-TFs gel. It was well tolerated by rat skin. Hence CPX-TFs gel could be the potential delivery system for transdermal administration of CPX for treatment of skin infections.
Introduction
Skin infections are the most prevalent disease amongst the various infectious diseases [1]. The largest organ in the body, the skin can make up 15% of the body’s weight. It carries out a variety of responsibilities, including protecting the body against physical, chemical, biological, and environmental dangers, preventing excessive water loss from the body, and controlling body temperature. Anatomically it has the epidermis, the dermis, and the subcutaneous layer [2]. Skin infections can range from moderate to severe life-threatening infections showing symptoms like a tiny patch of redness, swelling, pain, and erythema on the entire skin surface. These types of infections are becoming more common as the population ages as the number of critically ill patients and immune-compromised people increases. Emergence of multi-drug resistant microorganisms is another reason for it [3].
Antibiotics are given by topical, oral, or parenteral rout depending on the type of skin infection. In some special cases like surgical excision and drainage the administration of oral and intravenous antibiotics should be used to treat any additional infections. Otherwise topical therapy can be suggested for common cases of mild folliculitis and impetigo [4]. Oral antibiotics are usually sufficient for individuals with manageable co-morbidities and no evidence of systemic toxicity, but parenteral therapy is required in critically ill patients [5]. Aminoglycoside, polypeptide, cephalosporin, macrolide, penicillin, and quinolone are among the groups of medications that are frequently prescribed. Since the majority of antibiotic drugs in topical formulations do not penetrate deeper skin layers and subdermal tissues. As a result, oral or parenteral antibiotics in high doses are used to treat skin infections. This lead to unpleasant side effects including diarrhoea, nausea, nephrotoxicity, myotoxicity, myelosuppression, severe pancreatitis, mild thrombocytopenia, and with drug resistance. This leads to a necessity for the formulation of transdermal drug delivery systems (TDDS), which helps to address the issues with drug administration. TDDS alter the drug’s absorption, distribution, and elimination to improve the product’s effectiveness and boost patient compliance.
The lipid vesicles have been found to be beneficial in various areas such as immunology, membrane biology, diagnostic procedures, and in clinical therapy [6]. Hence the transdermal administration of lipid vesicles offers a reliable way to deliver drug to the infection site, reducing drug toxicity with little to no side effects. By improving drug bioavailability, particularly in the case of drugs that are poorly soluble, as well as by reducing dosage and frequency while improving patient compliance, it lowers the cost of therapy. They are capable of incorporating both hydrophilic and lipophilic drugs (Jain et al., 1997). A lipid vesicle called as TFs are made up of phospholipids and an edge activator, which are primarily non-ionic surfactants. Transferosomes squeeze along the stratum corneum to circumvent the obstacle of skin penetration [7]. The mixture of lipids and surface-active components in the optimized concentration makes the membrane of TFs more flexible. It can squeeze itself through a pore and deliver the drug through the skin barrier non-invasively without measurable loss. Controlling medication loss and deterioration, avoiding negative side effects, and enhancing drug availability at the site of the disease are the important properties of the ideal TDDS [8]. Hence the drugs can be encapsulated in vesicular structures like TFs to extend their time in the bloodstream and, possibly, lessen their toxicity [9,10].
According to reports, TFs penetrates the skin’s layers deeply, moves quickly through unbroken skin, and is absorbed into the systemic circulation [11]. It has been observed that TFs with a size between 200 and 300 nm can easily pass through skin [12]. Therefore, by creating drug loaded TFs and putting them into TDDS, many side effects can be avoided and the drug can reach the systemic circulation as well as the layers beneath the skin. For treatment of skin infection various antibiotics are used but the topical administration having drawback of lower penetration through the deeper layers of skin. Moreover the oral and parenteral administration of drug in large dose leads to the severe side effects. To overcome the problems associated with the drug (cephalexin) administration, there is need to develop potential transdermal delivery system. Delivery system containing transferosomes was found to be promising carrier to enhance the skin penetration and improved bioavailability. Lower particle size in nanometer range can be the other advantage. However, many studies on formulation of cephalexin such as self-nanoemulsifying drug delivery [13] and others were reported but the work on cephalexin loaded transferosomal gel was not published till date. Cephalexin transferosomal gel is innovative because of its distinct composition and possible benefits over other cephalexin delivery methods. In order to obtain the intended therapeutic effect, a lower dose of the drug may be needed, which lowers the chance of adverse effects. Hence the present study was designed to develop cephalexin loaded transferosomal (CPX-TFs) gel for an effective treatment of skin infection by improving transdermal delivery. Moreover the major objectives of the study was to prepare CPX-TFs with higher entrapment and lower particle size, to enhance the penetration deeper into the skin layers. The TFs were prepared by thin film hydration method by employing QbD based Box-Behnken design considering concentrations of phospholipon® 90H, sodium deoxycholate and temperature as independent variables and using these parameters formula was optimized.
Chemicals and reagents
Cephalexin was received from Aurobindo Pharma, Hyderabad, India. Phospholipon® 90H was obtained as a gretis from Lipoid GmbH, Germany. The supplier of sodium deoxycholate was Vishal Chemical in India. The supplier of carbopol 934 was Lubrizol Pvt Ltd. in India. Analytical-grade materials were employed for all other compounds and reagents.
Read more
Nilesh R. Rarokar, Suprit D. Saoji, Nishika V. Deole, Madhuri Gaikwad, Abhijeet Pandey, Chinnaperumal Kamaraj, Suresh V. Chinni, Vetriselvan Subramaniyan, Gopinath Ramachawolran, Sanket Dharashivkar, Preparation and formula optimization of cephalexin loaded transferosomal gel by QbD to enhance the transdermal delivery: In vitro, ex vivo and in vivo study, Journal of Drug Delivery Science and Technology, 2023, 104968, ISSN 1773-2247,
https://doi.org/10.1016/j.jddst.2023.104968.

