Pre and post characterization of ODTs with emphasis on compression force and quality of super-disintegrants: In vivo analysis in healthy volunteers
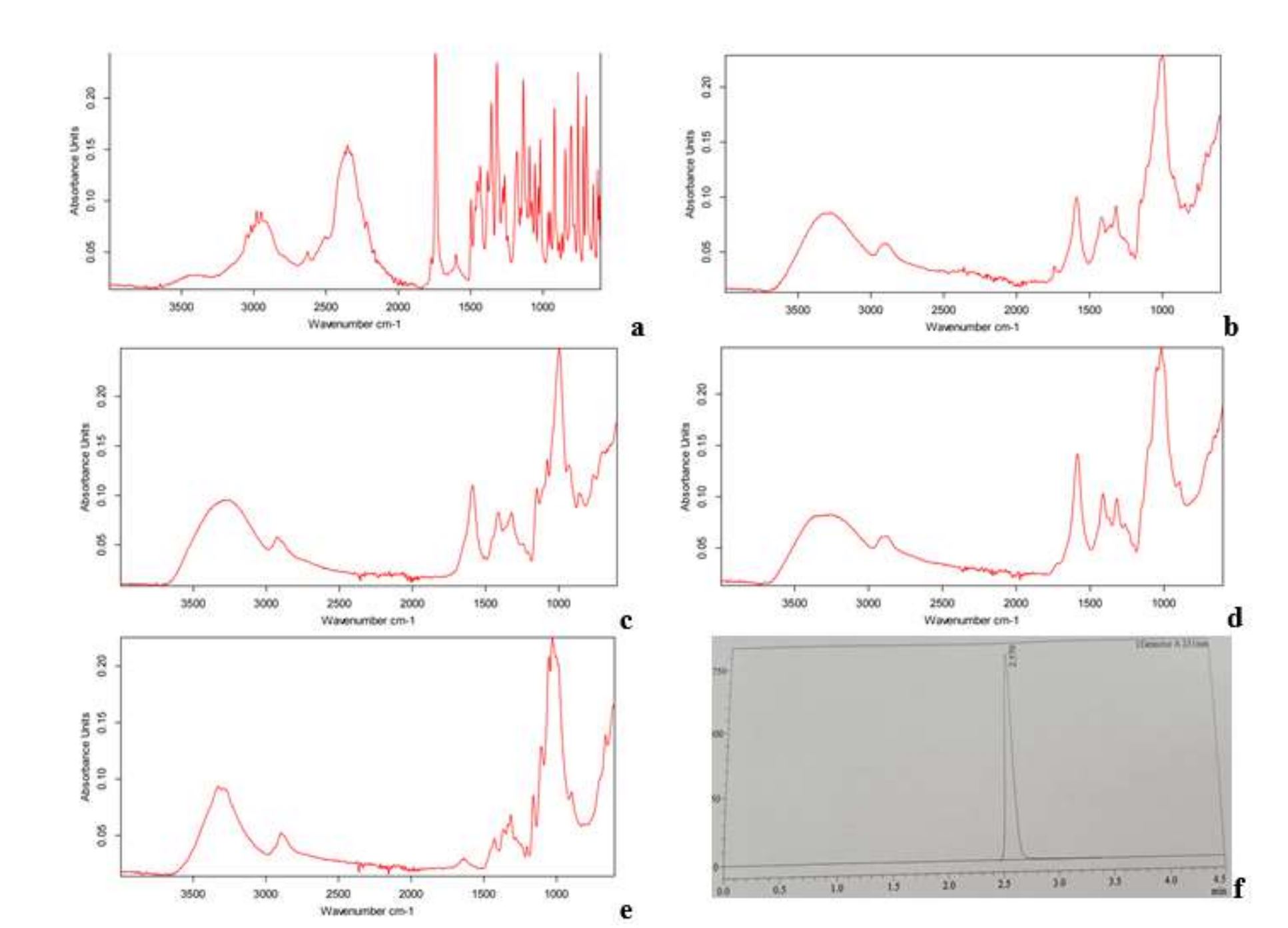
Oral dispersible tablets (ODTs) are patient compliant dosage forms which rapidly disintegrate in the mouth following active absorption with rapid onset of action. The current study was designed to resolve compression problems used for ODTs, as high compression force exhibited hardness and drug release problems. Formulations, F1-F9 were compressed at three different forces 44, 54, and 64 kN using cross-carmellose sodium (CCS) and sodium starch glycolate (SSG) and evaluated for pre and post compression. Formulations F1, F4 and F7 which were compressed at 44 kN showed hardness ranges between 5.09-6.15 with lowest DT (less than 15 s) and better LTZ release.
While F2, F5 and F8 (compressed at 54 kN) demonstrated hardness in between 6.90-7.02. Similarly, F3, F6 and F9 compressed at 64 kN showed hardness values between 8.70-8.98 with increased DT and slow LTZ release. Friability results for all the formulations were within United States Pharmacopeial (USP) specifications (<1%). All formulations depicted t-test value <0.5, hence it found that all formulations showed significant statistical value within limits, however best compression force 44 kN showed low p value. It was concluded that optimized compression force for ODTs was 44 kN among all employed forces that exhibited desirable drug release.
Download the full article as PDF here Pre and post characterization of ODTs with emphasis on compression force and quality of super-disintegrants
or read it here
Materials
The anti-histaminic drug was sourced from Titlis® Pharma (Pakistan). The polymer sodium starch glycolate (SSG) was purchased from Yung Zip Chemical Ind®, and croscarmellose sodium (CCS) were purchased from Mingtai Chemical® (Taiwan). Similarly, Aerosil® (Cabot®), microcrystalline cellulose (MCC), and dicalcium phosphate dihydrate were purchased from JRS Pharma® (Germany). Magnesium Stearate was purchased from Peter Ge® (Malaysia). Menthol was purchased from Anhui Great Oils® (China).
Pre and post characterization of ODTs with emphasis on compression force and quality of super-disintegrants: In vivo analysis in healthy volunteers, Shagufta Ayub, Sana Hanif, Umar Inzamam ULHuq, Muhammad Irfan, Ijaz Ali, Osama A. Madkhali, Kalsom Farzana, Muhammad Ali Syed6*, Nariman Shahid, Tayyaba Aftab2 and Maliha Asmatullah, November 2023Pakistan Journal of Pharmaceutical Sciences 36(6):1767-1775 DOI:10.36721/PJPS.2023.36.6.REG.1767-1775.1
Read more on Disintegrants – Pharmaceutical Excipients here and watch the video below:

