A Comprehensive Review of Disintegrants: Backbone of disintegration
Abstract
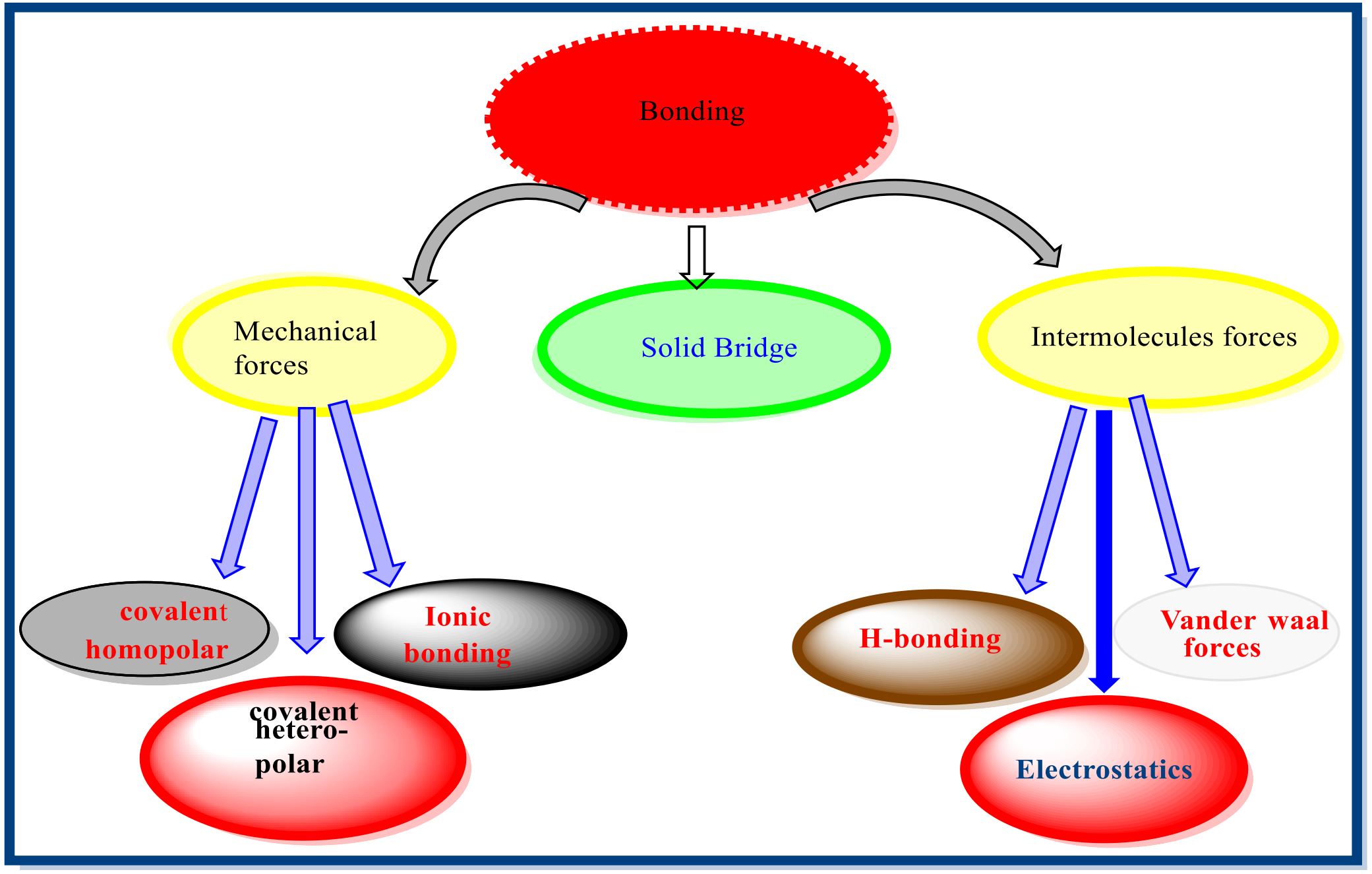
Strong attractive bonding forces works among the particles of solid dosage forms such as mechanical, solid and intermolecular and bioavailability of solid dosage form is preferentially dependent on in vivo disintegration then dissolution. This review is focused for disintegrating agents, their categories, mechanisms of action, associated pros and cons. Furthermore, more emphasis is on natural superdisintegrants, those perform disintegration at fastest rate with limited side effects. So, they are in frequent use to create number of preparations like fast dissolving tablet, pulsatile and tablet dispersible tablet etc. Despite of various disintegrants, superdisintegrants co-processed form of them are thoroughly investigated along with novel techniques of production such as Melt Extrusion, Crystallization, Spray Drying, Solvent Evaporation, Granulation/ Agglomeration.
Introduction
The Although, a variety of dosage forms are available but still oral dosage forms are more preferred by
humankind. As solid dosage forms are compact in structure that make them more acceptable dosage form.
Different bonding forces among solid particles work during the preparation of solid dosage forms, particularly
granules and tablets, to transform them into appropriate dosage forms. The mechanism followed by dominating
bond additionally, surface area across which these bonds are active could be regarded as the two main criteria
for the compactability of powders. These aspects have not been thoroughly assessed for medicinal materials due to significant experimental challenges. Instead, studies and relationships with tablet strength typically focus on
more auxiliary, indirect aspects. Such auxiliary elements as particle form, plastic deformation, surface
roughness, particle size and particle fragmentation, Elastic deformation and have all been examined as important
volume reduction methods [1-4].
The bonding mechanisms that have been discussed include mechanical interlocking, that represents the bond
kind which dependent on twisting and hooking of uneven particles in shape, solid bridges, which represent
uninterrupted solid bridges between tablet and granules particles, intermolecular forces, which represent infirm
attraction forces active over the distances. Computation of dense strength have been made in media that are
acknowledged to diminish intermolecular forces bonding in order to describe the dominant bond mechanisms.
Liquids with various dielectric constants and films made of magnesium stearate served as the media. The
findings show that the intermolecular forces are the primary type of bonding in medicinal materials. Bonding
with solid bridges can only improve compact strength of particles having coarse nature, plastically deforming
materials that could melt during compaction. Only for coarse plastically deforming materials that can melt
during compaction can bonding with solid bridges help to the compact strength. All bonding shown in (Figure
1).
Only a proportionate connection between the bonding surface area and compact surface area may be feasible for
all materials joining with intermolecular interactions [2]. If the particle surface area in the tablet is big, a huge
bonding surface area for such materials will be obtained. This could be done by using materials that are heavily
fragmented or qualities with noticeable surface roughness or by using very refined particles materials. It is
indicated that the majority of the supposed plastically deforming pharmaceutical materials frequently lack the
flexibility necessary to produce sizable zones that could participate in the attraction of different particles by
different molecules.
Download the full article as PDF here: A Comprehensive Review of Disintegrants: Backbone of disintegration
or read it here
7.2 SUPERDISNTEGRANT’S CATEGORIES
7.2.1 Superdisintegrants Obtained from Nature
Gumseg. Guar Gum, Xanthan Gum, Locust Bean, Cassia Fistula Gum, Karaya Gum,
Gellan Gum.
Agar eg. Gelidiumamansii
Chitosan eg β 2-amino-2-d-glucose
Soy polysaccharide Emcosoy
Superdisintegrants Made Synthetically
Modified starches eg. Sodium Starch Glycolate
Modified cellulose eg. Croscarmellose
Cross-linked poly-vinyl pyrrolidone eg. Crospovidone, polyvinyl-pyrrolidone
Modified Resin eg. Indion 414, Kyron 314
Microcrystalline Cellulose eg. Avicel 102
Cross-linked alginic acid eg. Alginic acid NF
L-substituted Hydroxypropyl derivatives.
Co-processed superdisintegrants
Eg Starlac (maize starch and lactose).
Starcap 1500 (pregelatinized starch and corn starch).
Ran Explo-C (silica, microcrystalline cellulose, and crospovidone).
Ludipress (crospovidone, lactose monohydrate and polyvinylpyrrolidone).
PanExcea MH300G (microcrystalline cellulose, crosspovidone and hydroxyl- propyl- methyl cellulose).
Ran Explo-S (microcrystalline cellulose, sodium starch glycolate and silica).
Following excipients are mentioned in the study besides other: Lycatab® or Sepistab®, Vivapur 101®, silicon dioxide, Polacrilin potassium
Monika Sharma, Amarjeet Singh, Sonia Gupta, Suresh Kumar, Sandeep Kumar and Ankur, A Comprehensive Review of Disintegrants: Backbone of disintegration, Latin American Journal of Pharmacy, Lat. Am. J. Pharm. 43 (1): (2024), ISSN 0326-2383, website http://actafarmbonaerense.com.ar/index.php/latamjpharm/article/view/866
See also our introduction article on Disintegrants:


