Production challenges of tablets containing lipid excipients: Case study using cannabidiol as drug model
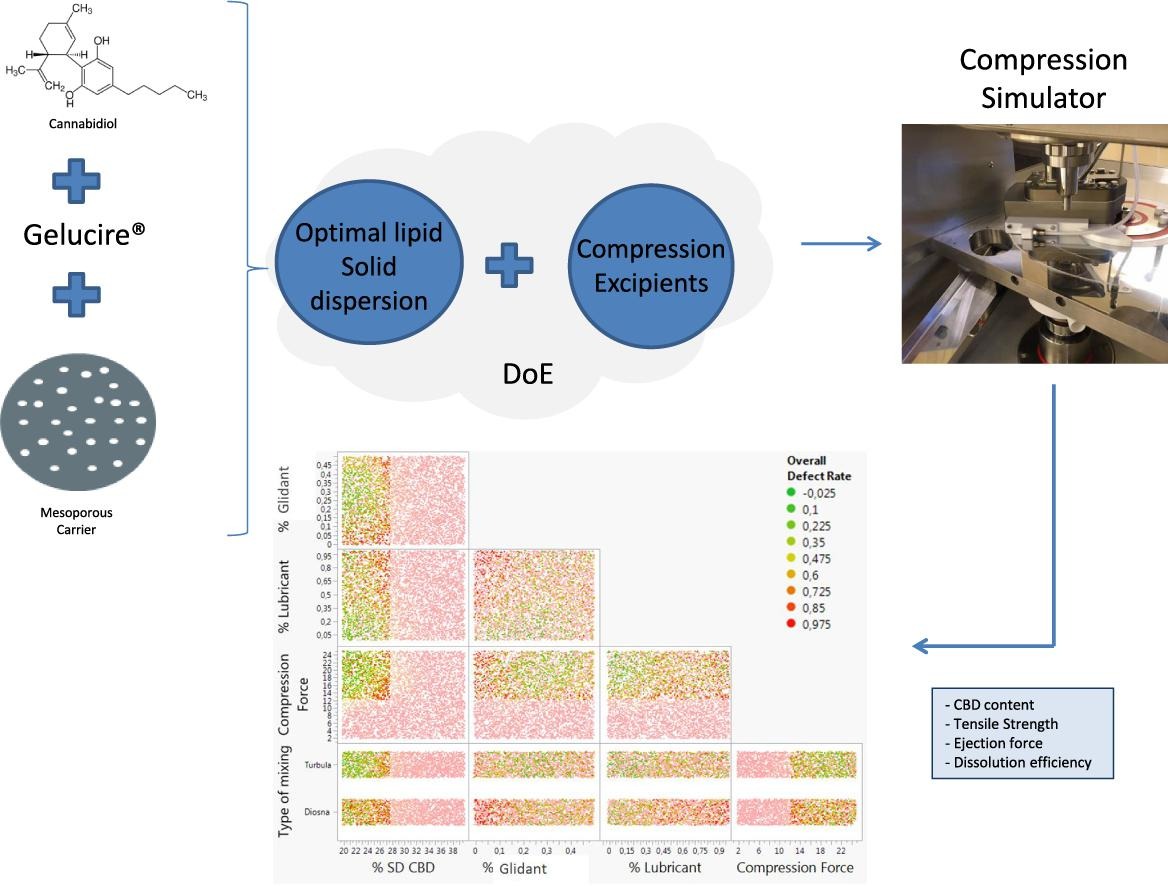
The aims of this study were, firstly, to select an optimal lipid solid dispersion of cannabidiol among different lipid excipients (Gelucire® 50/13, 48/16, 44/14 and Labrasol®) and inorganic carriers (colloidal silica, Syloid® XDP and Neusilin® US2) through a screening plan. The enhancement of aqueous solubility of cannabidiol from a free-flowing powder with adequate drug content was obtained by mixing cannabidiol (20%) with Gelucire® 50/13 (40%; Gattefossé, France), both incorporated inside mesopores of mesoporous silica Syloid® XDP (40%; Grace, Germany).
Secondly, we have studied the tableting properties of this selected dispersion through a Design of Experiments (DoE) by manufacturing tablets with other excipients with using a compression simulator (Styl’One® Evo, MEDELPHARM, France). The design of experiments included the percentage of lipid solid dispersion, of glidant, of lubricant and different compression forces. The dissolution efficiency, the drug content, the tensile strength and the ejection force were analyzed. The DoE showed that % of dispersion as well as compression forces were the main influential variables. An exit of lipid materials outside the mesopores of silica due to compression process has been highlighted, reflected by reduced tensile strength.
This study showed the possibility of manufacturing tablets with lipid materials even if limitations have been highlighted. Indeed, the dispersion percentage must not exceed 27% and compression forces up to 13 kN are required to produce lipid tablets with optimal properties.
Read more
The compression simulator Styl’One® Evo from MEDELPHARM was used in the study.
Nathan Koch, Olivier Jennotte, Céline Toussaint, Anna Lechanteur, Brigitte Evrard, Production challenges of tablets containing lipid excipients: Case study using cannabidiol as drug model, International Journal of Pharmaceutics, Volume 633, 2023, 122639, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.122639.

