Development, QbD based optimization and in vitro characterization of Diacerein loaded nanostructured lipid carriers for topical applications
Abstract
Objective
DCN is a chondro-protective agent which displays inadequate oral bioavailability along with gastrointestinal side effects. It is a choice of drug for treatment of osteoarthritis. Therefore, the objective of this study was to develop and optimize DCN loaded nanostructured lipid carrier (NLCs) for topical delivery.
Method
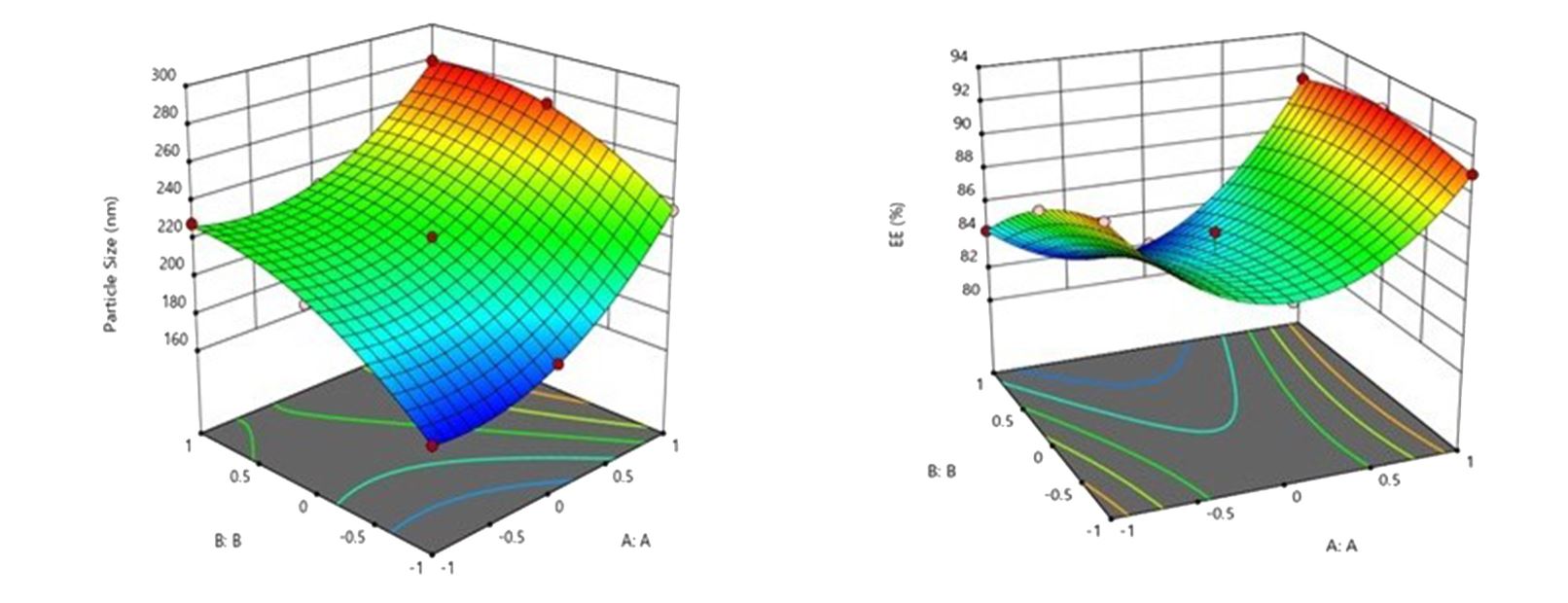
NLCs were prepared by hot homogenization followed by sonication method. The formulation was optimized by 2 factor 3 level central composite design taking two independent variables i.e. concentration of stearic acid (A) and concentration of oleic acid (B), and three independent variables, particles size (Y1), % entrapment efficiency (Y2) and % drug release at 24 hours(Y3).
Result
The particle size, % entrapment efficiency and in-vitro drug release at 24 hours of optimized formulation was found 173.76 nm, 91.30%, and 94.17% respectively. The optimized DCN-NLC formulations were analyzed by DSC and XRD, TEM, and FTIR studies. DCN-NLC loaded gel was prepared and characterized for physical examination, viscosity and stability parameters. DCN-NLC in the gel form displayed sustained drug release and significant (p<0.05) tissue penetration in vitro in comparison to plain gel and DCN solution. The drug release kinetics study illustrated the Higuchi’s release pattern for prolonged period of time. Ex vivo permeation study result supported good topical penetration for DCN using DCN-NLCs.
Conclusion
Conclusively, the developed formulation has potential for topical application for future pre-clinical studies.
Download the full article as PDF here: Development, QbD based optimization and in vitro characterization of Diacerein loaded nanostructured lipid carriers for topical applications
or read it here
Materials
DCN was purchased from Quingdao Sigma Co. Ltd. China. The other chemicals used in this study such as ethanol, methanol, disodium hydrogen orthophosphate, potassium di-hydrogen orthophosphate, stearic acid, oleic acid, Tween 80, and Carbopol 940 were purchased from Loba Chemicals Mumbai, India
Disha Kesharwani, Swarnali Das Paul, Rishi Paliwal, Trilochan Satapathy, Development, QbD based optimization and in vitro characterization of Diacerein loaded nanostructured lipid carriers for topical applications, Journal of Radiation Research and Applied Sciences, Volume 16, Issue 2, 2023, 100565, ISSN 1687-8507, https://doi.org/10.1016/j.jrras.2023.100565.
Read more on ODT here


