The influence of hydrogen bonding between different crystallization tendency drugs and PVPVA on the stability of amorphous solid dispersions
Abstract
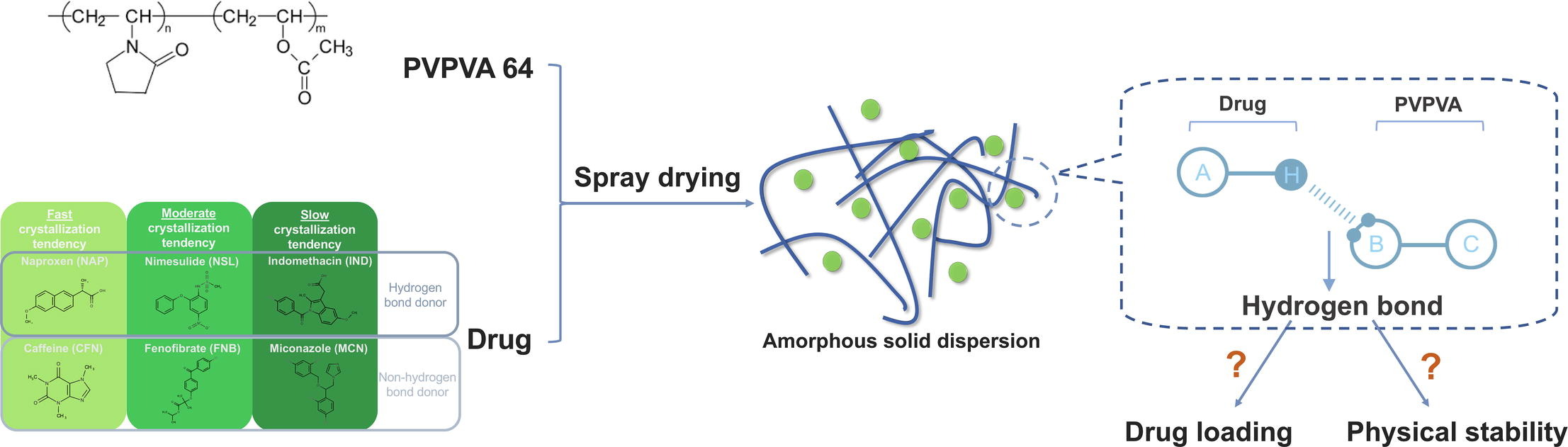
Amorphous solid dispersion (ASD) is one of the formulation strategies for drugs displaying low solubility and low oral bioavailability. In this study, high drug-loaded ASDs of drugs with different crystallization tendencies were prepared by spray drying. The aim was to investigate the influence of hydrogen bonding between the drug and the model polymer PVPVA on the physical stability of ASDs containing drugs with different crystallization tendencies. From the 60-day stability study results, the intermolecular hydrogen bonding has a considerable stabilizing effect on the ASDs of the drug with a moderate crystallization tendency. Nimesulide (hydrogen bond donor) can maintain the amorphous form for a longer time than Fenofibrate (no-hydrogen bond donor) during storage. In the ASDs with fast crystallization tendency drugs (naproxen and caffeine), intermolecular hydrogen bonds are not very effective in preventing drug crystallization, and the effect on the stability of ASD is relatively weak.
However, for drugs with a slow tendency to crystallize (indomethacin and miconazole), the ASDs remained in an amorphous state during the monitored storage period, making it impossible to compare the effect of intermolecular hydrogen bonds on the stability of this type of ASDs. It also reveals that intermolecular hydrogen bonds can increase the drug loading capacity of ASDs. The relationship between drug loading and ASD stability was further analyzed by the state diagram. This study clearly pointed out that the physical stability of ASDs of drugs with different crystallization tendencies is affected to a different extent by intermolecular hydrogen bonds.
Introduction
Most drug candidates with development potential are BCS II and BCS IV compounds, and their poorly water-soluble characteristics greatly challenge the process of drug development. Experts have tried many strategies to solve this problem, amorphous solid dispersions being one of the most widely used strategies today. Formulating the poorly water-soluble drug with a polymer into an amorphous solid dispersion, improves the dissolution rate and solubility by reducing the energy required for these processes, while the polymer can decrease the molecular mobility, inhibiting drug nucleation and crystal growth, and prolong the time to maintain a supersaturated solution in the gastrointestinal fluids to a certain extent (Janssens et al., 2009, Sun and Lee, 2015).
However, since ASDs have higher free energy than stable crystalline forms, they are thermodynamically less stable. The stability of ASD system can be better understood and designed through the state diagram. As the state diagram shown in Figure 1,the green curve shows the glass-transition temperature of the ASD, while the blue curve shows the solubility of the drug in the polymer. If the drug loading is lower than the solubility, the ASD system is in the thermodynamic stable region above the solubility line (Fig. 1 I and II regions), and will not crystallize. While the glass-transition temperature of the ASD affects the kinetic stability of the system, when the storage temperature is low enough relative to the glass-transition temperature, an ASD system with adequate kinetic stability can be obtained (Fig. 1 II and III regions) (Lehmkemper et al., 2018, Lehmkemper et al., 2017; Manfred Gordon and Taylor, 1952, Prudic et al., 2014). Generally, ASDs with a high glass transition temperature (Tg) are considered to have better stability, thus the polymers with high Tg are usually selected as carriers for ASDs preparation to increase the Tg of the amorphous system (Alhalaweh et al., 2015, Anderson, 2018, Graeser et al., 2009, Janssens and Van den Mooter, 2009, Kyeremateng et al., 2014). For improving the therapeutic compliance, ASD systems with higher drug loading are more inclined to be prepared, however, the position of ASDs with high drug loading in the state diagram is closer to the critical value of the solubility, which means that the drugs have higher tendency to phase separate and transform to the thermodynamically stable state during long-term storage. The potential instability of high-drug loaded ASD systems greatly limits the development of ASD.
Besides, previous studies have shown that hydrogen bonding as one of the intermolecular interactions between the drug and polymer has a considerable effect on improving the stability of ASD systems. Taylor et al. showed that hydrogen bonds formed between Indomethacin and PVP affect the crystallization kinetics of Indomethacin, preventing crystallization (Taylor & Zografi, 1997). Sarpal et al. demonstrated that the hydrogen bonding between the drug and the polymer affects the phase homogeneity of the ASD system, and that increasing the H-bonding propensity of the polymer does lead to a more homogeneous system, which in turn improves the stability of the system (Sarpal et al., 2019). In another investigation, ASDs with stronger drug-polymer hydrogen bonding interactions exhibited longer relaxation times and lower molecular mobility, which further influenced the drug crystallization kinetics (Kothari et al., 2015). Another important factor that dictates the physical stability of ASDs is the intrinsic crystallization tendency of the drug. Baird et al. and Van Eerdenbrugh et al. has grouped APIs according to their crystallization tendency. The APIs were classified as rapid, moderate, and slow crystallizers according to their different tendency to transit from an amorphous state to the crystalline state. They evaluated the correlation between crystallization tendency of the drug and glass forming ability (GFA). The GFA is related to the stability of ASDs, so the crystallization tendency of the drug may have a certain correlation with stability of ASDs (Baird et al., 2010, Van Eerdenbrugh et al., 2010).
Spray drying (SD) is one of the most common techniques for preparing ASDs, due to its advantages of efficient, continuous and scalable manufacturing (Guns et al., 2011, Singh and Van den Mooter, 2016, Tambe et al., 2022, Ziaee et al., 2017). Spray drying is a solvent based method where a solution containing drug and polymer is transformed to a powder using a heated gas, usually air. The drug is physically trapped in the molecular state in the polymer matrix which prevents the drug from nucleation and crystallization. In addition to the influence of the preparation process and the internal factors of the ASD system on the stability, the temperature and humidity of the storage environment also have a considerable impact on the stability of the ASD system. The humidity and temperature in the environment can facilitate amorphous–amorphous phase separation and/or crystallization of drugs from ASDs, which leads to physical instability of ASDs (Alhalaweh et al., 2015, Graeser et al., 2009, Rumondor et al., 2009, Rumondor and Taylor, 2010).
Therefore, this study aimed to investigate the extent to which hydrogen bonding affects the physical stability of ASDs prepared from drugs with different crystallization tendencies. The spray dried samples were stored at different temperature and relative humidity (RH) for investigating the impact of storage conditions on the stability of ASDs. Samples were analyzed for crystallinity by modulated differential scanning calorimetry (mDSC) and x-ray powder diffraction (XRPD). In the study, six model drugs were selected according to three crystallization trends. There are two drugs in the same crystallization trend group, one contains a hydrogen bond donor and the other does not contain hydrogen bond donor. Therefore, in this experiment, naproxen (NAP) with hydrogen bond donor and caffeine (CFN) without hydrogen bond donor were selected as fast crystallization model drugs. Nimesulide (NSL) was selected as the moderate crystallization model drug with hydrogen bond donor and fenofibrate (FNB) as a moderate crystallization model drug without hydrogen bond donors. Indomethacin (IND) and miconazole (MCN), a drug with a low Tg (ca. 1°C) were selected as slow crystallization model drugs with and without hydrogen bond donors, respectively. Due to the characteristics of PVPVA, such as high glass transition temperature, good solubility in dichloromethane (DCM), the presence of only hydrogen acceptor groups, this polymer was employed as model polymer in this study.
We also determined the Tg of the different ASDs as a function of the composition as well as the depression of the melting point (Tm) of the drug in the presence of the polymer.
This study deeply explored the differences in the effect of hydrogen bonding between drug and polymer molecules on the physical stability of ASDs containing drugs with different crystallization tendencies. The conclusion obtained can give more guidance for the ASD formulation design of drugs with different crystallization tendencies.
Read more here
Materials
Naproxen (NAP) was purchased from SA Fagron NV (Waregem, Belgium) and caffeine (CFN) was purchased from Sigma-Aldrich (Overijse, Belgium). Nimesulide (NSL) was supplied by LKT Laboratories (St. Paul, Minnesota, USAs) and fenofibrate (FNB) was obtained from Hangzhou Dayangchem Co. (Hangzhou City, China). Indomethacin (IND) was received from Alfa-Aesar (Kandel, Germany) and miconazole (MCN) was obtained from Janssen Pharmaceutica N.V. (Beerse, Belgium). Poly(vinylpyrrolidone-co-vinyl acetate) 64
Jingya Wu, Guy Van den Mooter, The influence of hydrogen bonding between different crystallization tendency drugs and PVPVA on the stability of amorphous solid dispersions, International Journal of Pharmaceutics, 2023, 123440, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123440.
See the webinar:
“Fast Track development of Biphasic nano-dexamethasone Pellets using galenIQ™”, 12 October 2023:
Get more information & register here for free:


