Advanced analysis of disintegrating pharmaceutical compacts using deep learning-based segmentation of time-resolved micro-tomography images
Abstract
Introduction
Pharmaceutical tablet disintegration, the process by which a tablet breaks apart into fragments in the desired manner upon initial contact with gastrointestinal fluids, is crucial for the bioavailability of drugs and ensuring consistent release behavior of the active pharmaceutical ingredient (API) for modified release formulations. For fast-releasing tablets, the desired disintegration profiles are achieved by ensuring rapid fragmentation of the compact to increase the surface exposed to the dissolution medium. The rate and extent of fragmentation need to be precisely limited for slow-release formulations. Despite its importance, understanding of the disintegration mechanism remains incomplete [[1]].
Many approaches have been used to characterize the processes proposed to play a role in overcoming the inter-particle forces that hold the compact together and thus lead to disintegration [[1],[2]]. In the common understanding, disintegration starts with and is sustained by liquid penetration into the porous domain of the tablet [[3],[4]]. Therefore, the compact’s porosity plays an important role, as well as the presence of a percolating network of material that conducts water throughout the tablet for rapid disintegration. One such water transportation process is called wicking [[5]]. Wicking is followed by swelling, a commonly accepted aspect of the disintegration mechanism whereby a disintegrant polymer omnidirectionally expands, with water acting as a plasticizer [[6],[7]]. Additionally, unidirectional elastic recovery of deformation-induced strains upon contact with water has been put forward as a mechanism of action for certain disintegrants [[8],[9]].
Other, more contended disintegration mechanisms have been proposed, for example, pressure buildup in the porous domain resulting from entrapped air heating up due to wetting of the pores [[1],[10]]. Another controversial disintegration mechanism hypothesized for starches is particle-particle repulsion resulting from water attenuating hydrogen bonds, van der Waals and electrostatic forces, and subsequent induction of a repulsive force between the particles [[11]]. The complexity of the disintegration mechanism and difficulty quantifying it arise from the complex interplay of different formulation components with various functions based on their physicochemical properties. These properties must be investigated in isolation, combined with the other formulation components, and with the disintegration medium to quantify their effect.
So far, the quantification of disintegration was either based on superficial observation, static measurements, or indirect detection of the processes occurring within the tablet. Experiments include, for example, simultaneous water uptake rate and disintegration force measurements [[12]]; direct pore network imaging using micro-computed tomography (μCT) [[13],[14]]; an indirect measure of bulk compact porosity using terahertz time-domain spectroscopy [[15],[16]]; application of high-speed video recording to visualize disintegrant in action [[8]]; application of high-speed MRI imaging to quantify water distribution and swelling during the disintegration process [17, 18, 19, 20, 21]; methods to predict disintegration behavior based on density distribution measurements using ultrasound amongst other compact properties [[22]]; water-front and internal defect measurements using terahertz pulsed imaging [[23],[24]]; characterization of compact fragments resulting from disintegration using focused beam reflectance measurements [[25],[26]]; dynamic laser diffraction [27, 28, 29]; or video recordings [[8],[30]]. Existing research thus covers many aspects of this complex process. Due to the process’s complexity, interpreting indirectly obtained or isolated results can often lead to misinterpretation of the underlying mechanism. Time-resolved X-ray micro-computed tomography is an imaging technique that acquires direct visual data at high spatial and temporal resolutions, making it possible to observe and quantify the complete process.
Static X-ray μCT [31, 32, 33] is a powerful, non-destructive, high-resolution volumetric imaging technique that provides valuable insight into the internal microstructure of visually opaque samples. Thus, μCT is applied across a broad range of research fields, from earth science [[34]], medical research [[35]], and material science [[36]] to pharmaceutical research [[37],[38]]. The use of synchrotron radiation [[32]] enables phase-contrast imaging [[39]]. Resolutions in the submicron [[40]] down to the nanometer (i.e., less than 100 nm) ranges [[41]] become attainable thanks to the parallel, coherent, and high-brilliance X-rays produced by synchrotron facilities. The high flux of these X-ray sources also provides the possibility of acquiring time-resolved image sequences of dynamic processes through the rapid and consecutive acquisition of CT scans, i.e., time-resolved μCT.
Time-resolved μCT has been applied to study foam systems [[42],[43]], fluid interactions with porous geological samples [[44],[45]], or to observe the mechanics involved in a wingbeat of the blowfly [[46]]. Further extending visual analysis with computer-aided techniques requires consistent segmentation of the dynamic volumetric data, which needs consistent imaging quality throughout the entire acquisition. Enhancing the image quality of μCT imaging in second- or sub-second time regimes [[47]] is possible with techniques like phase-contrast enhancement [[48]]. Such strategies are essential at high acquisition rates to reduce the dose imparted by the X-rays to levels that will not damage the sample [[49]]. Sometimes, polychromatic X-ray radiation is preferable over monochromic to reach the flux intensity necessary for adequate image quality despite the beam hardening artifacts [[34]].
The appearance of motion artifacts is a known problem for time-resolved μCT. Any sample motion during a given CT acquisition step violates the base assumption of reconstruction algorithms that the sample remains static [[50]], thus introducing motion artifacts during reconstruction of the three-dimensional (3D) image out of the projection raw data. Simply increasing the acquisition rate is rarely viable due to the limitations coming from insufficient beam intensity [[49]]. Higher acquisition frequencies could also introduce distortions in the studied process as rotation-related forces constantly act on the sample during image acquisition [[34]]. The data volumes produced are another challenge, as higher acquisition rates generate several gigabytes per second of data streams. Therefore, specialized hardware and software are required for data acquisition, transfer, storage, image reconstruction, and analysis [[51]].
Image analysis, in general, plays an important role in various pharmaceutical applications, most prominently in quality control for manufacturing pharmaceutical dosage forms [[52]]. Examples include optical imaging as a non-destructive alternative to established techniques for predicting tablet hardness and other quality attributes of pharmaceutical compacts [[53]]. Optical imaging has also been applied to rapidly assess coating thickness on pellets by quantifying the color information of a dye in the coating layer [[54]]. X-ray-based imaging can be used as a non-destructive way of semi-quantitatively determining drug content by measuring the distribution and content of pellets within multiparticulate tablets [[55]] or by studying the drug particle distribution within tablets [[37]]. However, fully automated image analysis of complex objects, such as pharmaceutical tablet formulations during disintegration, remains an unsolved challenge for which approaches based on machine learning have been a promising development.
Machine learning applications in image analysis are slowly entering pharmaceutical research [56, 57, 58]. Deep learning [[59]], in particular, is a tool that has been successfully employed in computer vision tasks to overcome the severe limitations of standard image analysis, especially in terms of object recognition, image data classification, and feature extraction from complex and heterogeneous datasets. Medical imaging is a field where the application of deep learning shows promising results. Some recent applications include the classification of optical images as an automated skin cancer diagnostic tool [[60]], in radiology for automated breast cancer screening [[61]], and in predicting the progression of the severity of COVID-19 in patients based on computed tomography scans [[62]]. In all these examples, researchers reported that the diagnostic performance of the deep-learning algorithms was similar to or better than that of trained professionals.
Convolutional Neural Networks (CNNs) [[63],[64]] are a type of deep learning algorithm specialized in image processing using multiple consecutive layers of convolution operations for visual feature extraction. Image classification networks, like the GoogleNet Inception v3 CNN architecture [[65]], use these features for classification by coupling the output of the convolutional layers to a fully connected layer with an activation function. Similarly, image segmentation network architectures like the U-Net [[66]] connect the result of the convolution layers to a set of consecutive deconvolution layers. These then use the extracted feature information to build up a segmented version of the input image. Convolution operations make CNNs uniquely suited for and thus widely applied in image analysis, as the convolutions inherently process spatial information in addition to color information. CNNs can be adapted to use 3D convolution operations, which allows for the processing of 3D images from computed tomography scans [[67]], making CNNs a natural choice for the post-processing of μCT data.
Thus far, CNNs have been successfully applied to analyze static μCT data. Examples include the automated high-throughput extraction of morphological traits from μCT images of rice plants [[68]], geological sample classification [[69]], the classification of urinary stones from medical μCT scans [[70]], permeability estimation of complex pore structures of carbonate rock [[71]], and the quality enhancement of low-dose μCT scans [[72]]. In pharmaceutical research, CNNs have been applied to predict mini-tablet dissolution performance based on film coating integrity analysis using convolutional neural networks [[73]].
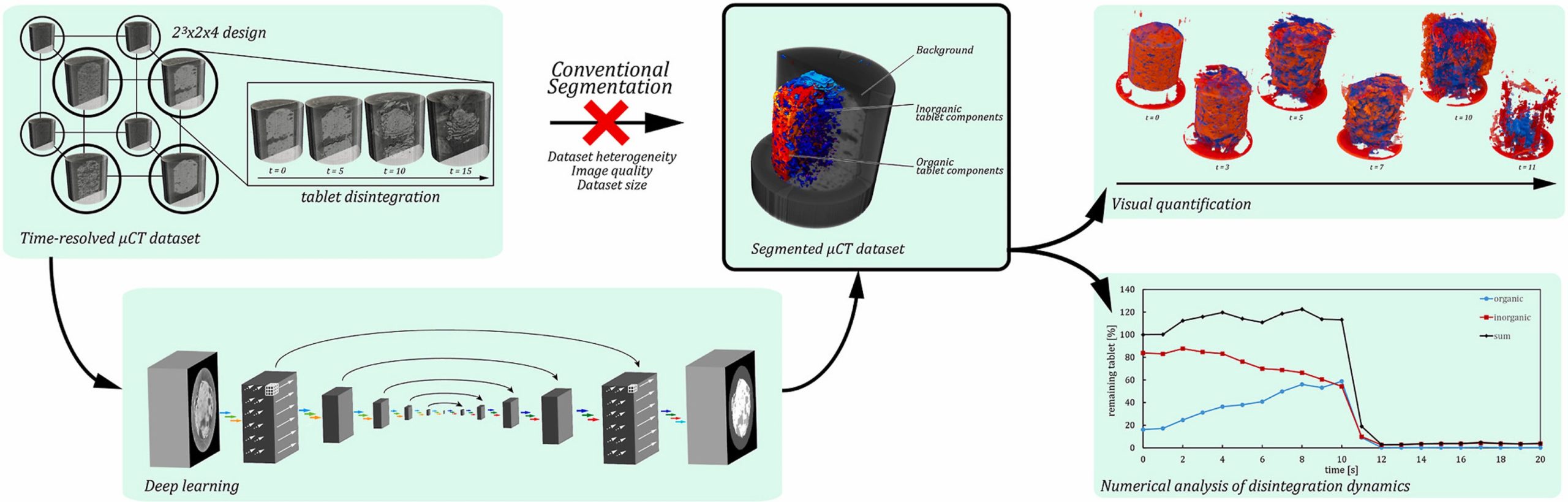
In this work, we extend the application of CNNs to the post-processing of dynamic μCT data. We prepared a comprehensive set of pharmaceutical tablets and acquired time-resolved μCT data that captured the disintegration of each formulation individually. CNN-based image segmentation allowed us to apply computer-aided and human expert-assisted analysis to the data and to illustrate our findings with videos showing the internal structures of each tablet during disintegration. We use factor and response surface analysis to assess the influence of formulation component properties on disintegration, and we relate the results of our direct measurements of tablets to established research. We provide our processed time-resolved μCT dataset for further research and our full software pipeline to make it easy to apply this imaging technique to other areas of pharmaceutical research.
Download the full article as PDF here: Advanced analysis of disintegrating pharmaceutical compacts using deep learning-based segmentation of time-resolved micro-tomography images
or read it here
Materials
The following compounds were chosen for their widespread use and their representative physicochemical properties: Caffeine (HPLC grade, Sigma-Aldrich, Switzerland), oxantel pamoate (Megafine Pharma Ltd., India), croscarmellose sodium (Ac-Di-Sol SD-711, FMC Corporation, Pennsylvania, USA), sodium starch glycolate (EXPLOTAB, JRS Pharma, Germany), hydroxypropyl methylcellulose (Methocel E4, Prochem, Switzerland), polyethylene oxide (WSR 301, Colorcon, Pennsylvania, USA), magnesium stearate (Hänseler AG, Switzerland), sodium stearyl fumarate (LubriSanaq, Pharmatrans Sanaq, Switzerland), (Fuji Chemical Industries Co., Ltd., Japan), Mannitol (Parteck M 300, Merck, Germany), microcrystalline cellulose (Avicel 102, FMC BioPolymer, Germany) and functionalized calcium carbonate (OMYAPHARM OG, OMYA AG, Switzerland).
Samuel Waldner, Erwin Wendelspiess, Pascal Detampel, Christian M. Schlepütz, Jörg Huwyler, Maxim Puchkov, Advanced analysis of disintegrating pharmaceutical compacts using deep learning-based segmentation of time-resolved micro-tomography images, RESEARCH ARTICLE, VOLUME 10, ISSUE 4, E26025, FEBRUARY 29, 2024, Published: February 12, 2024DOI:https://doi.org/10.1016/j.heliyon.2024.e26025
Read also our introduction article on Sodium Stearyl Fumarate here:


