In vitro-in vivo relationship for amorphous solid dispersions using a double membrane dissolution-permeation setup
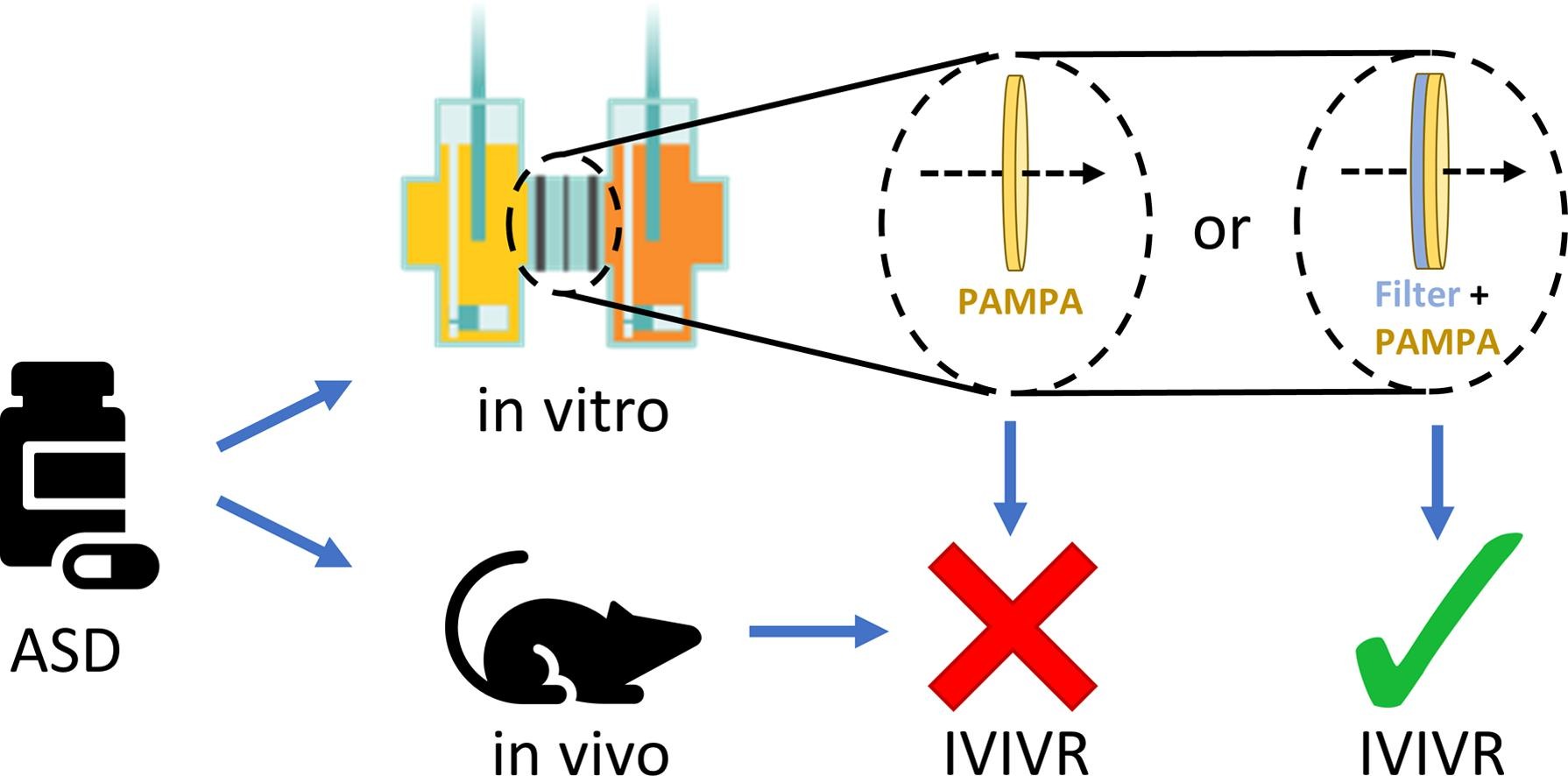
The use of amorphous solid dispersions (ASDs) is one commonly applied formulation strategy to improve the oral bioavailability of poorly water-soluble drugs by overcoming dissolution rate and/or solubility limitations. While bioavailability enhancement of ASDs is well documented, it has often been a challenge to establish a predictive model describing in vitro-in vivo relationship (IVIVR). In this study, it is hypothesized that drug absorption might be overestimated by in vitro dissolution-permeation (D/P)-setups, when drug in suspension has the possibility of directly interacting with the permeation barrier. This is supported by the overprediction of drug absorption from neat crystalline efavirenz compared to four ASDs in a D/P-setup based on the parallel artificial membrane permeability assay (PAMPA). However, linear IVIVR (R2 = 0.97) is established in a modified D/P-setup in which the addition of a hydrophilic PVDF-filter acts as a physical boundary between the donor compartment and the PAMPA-membrane. Based on microscopic visualization, the improved predictability of the modified D/P-setup is due to the avoidance of direct dissolution of drug particles in the lipid components of the PAMPA-membrane. In general, this principle might aid in providing a more reliable evaluation of formulations of poorly water-soluble drugs before initiating animal models.
Download the full article as PDF here In vitro-in vivo relationship for amorphous solid dispersions using a double membrane dissolution-permeation setup
or read it here
Materials
Efavirenz (EFV) was purchased from Jianbei Pharmaceutical (Zhejiang, China). Two grades of hypromellose acetate succinate (HPMCAS) (Shin-Etsu AQOAT® AS-LF and -MF) were supplied by Shin-Etsu Chemical (Tokyo, Japan). Polyvinyl alcohol (PVOH, GOHSENOL™ EG-03PW) was supplied by Harke Pharma (Mülheim a. d. Ruhr, Germany). Polyvinylpyrrolidone vinylacetate (PVPVA64, Kollidon® VA64) was donated by BASF (Ludwigshafen, Germany). PAMPA-membranes were prepared with hydrophobic polyvinylidene fluoride (PVDF) filters with a pore size of 0.45 µm from Frisenette (Knebel, Denmark) impregnated with gastrointestinal tract (GIT) lipid from Pion Inc. (Billerica, MA, USA). Sodium lauryl sulfate (SLS) and 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Hank’s Balanced Salt Solution (HBSS, 10x) was acquired from Thermo-Fisher (Waltham, MA, USA).
Jacob Rune Jørgensen, Wolfgang Mohr, Matthias Rischer, Andreas Sauer, Shilpa Mistry, Thomas Rades, Anette Müllertz, In vitro-in vivo relationship for amorphous solid dispersions using a double membrane dissolution-permeation setup, European Journal of Pharmaceutics and Biopharmaceutics, Volume 188, 2023, Pages 26-32, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2023.04.026.
Visit our new Webinar:
Solving capping challenges using mannitol as an excipient model
Get more information & register here:


