Drinkable in situ-forming tough hydrogels for gastrointestinal therapeutics
Abstract
Pills are a cornerstone of medicine but can be challenging to swallow. While liquid formulations are easier to ingest, they lack the capacity to localize therapeutics with excipients nor act as controlled release devices. Here we describe drug formulations based on liquid in situ-forming tough (LIFT) hydrogels that bridge the advantages of solid and liquid dosage forms. LIFT hydrogels form directly in the stomach through sequential ingestion of a crosslinker solution of calcium and dithiol crosslinkers, followed by a drug-containing polymer solution of alginate and four-arm poly(ethylene glycol)-maleimide. We show that LIFT hydrogels robustly form in the stomachs of live rats and pigs, and are mechanically tough, biocompatible and safely cleared after 24 h. LIFT hydrogels deliver a total drug dose comparable to unencapsulated drug in a controlled manner, and protect encapsulated therapeutic enzymes and bacteria from gastric acid-mediated deactivation. Overall, LIFT hydrogels may expand access to advanced therapeutics for patients with difficulty swallowing.
Main
The oral route provides a safe, rapid and facile course for drug administration, and results in greater patient comfort and compliance compared with parenteral routes1,2. Due to advantages in stability, dose consistency and the capacity to co-formulate with excipients, oral solid drugs have become the predominant formulation: they consistently account for ∼50% of new US Food and Drug Administration (FDA)-approved drugs (https://www.fda.gov/), and nearly 70% of Americans are on at least one prescription drug3. However, certain patient populations struggle with swallowing solids. More than 50% of children are unable to swallow standard-sized pills or capsules4. Patients with dysphagia, or difficulty swallowing, similarly struggle with solid drug forms. In adults, the prevalence of dysphagia can be as a high as 16%, and upwards of 37% have difficulty swallowing pills5,6. This may cause patients to skip or modify (for example, crush) their medications, which may result in altered pharmacokinetic profiles and death7.
While liquid formulations are easier to ingest, they are susceptible to rapid dilution within the gastrointestinal tract and are unable to spatially localize drug with excipients8, which challenge efforts to orally deliver biological drugs. A system capable of a programmed liquid-to-solid transition within the stomach could bridge the advantages of these two forms. A solid matrix could facilitate spatial proximity of drug and excipients that modulate drug release or protect drug activity against the harsh gastric environment, and augment gastric residence of a drug depot. Efforts to develop liquid-to-solid systems have relied on drinkable hydrogel systems crosslinked by calcium. Orally administered calcium carbonate-loaded alginate solutions undergo gelation in the stomach due to acid-triggered release of Ca2+ ions and subsequent crosslinking of alginate9. Similarly, gellan or alginate solutions mixed with complexed calcium undergo in situ gastric gelation10. Oral administration of an alginate/karaya gum solution followed by a solution of CaCl2 results in gelation in the stomach11. An antiacid medication comprising alginate, sodium bicarbonate and calcium bicarbonate has also been described12. However, these single-network hydrogels are mechanically weak and may not be able to withstand compressive forces within the stomach (up to 13 kPa)13, resulting in irreversible deformation and potential breakage of the formulation.
In this Article, we describe a new strategy to enable a drinkable, liquid in situ-forming and tough (LIFT) hydrogel, which comprises both ionic (calcium/alginate) and covalent (poly(ethylene glycol) (PEG)) polymer networks for enhanced toughness14. LIFT hydrogels undergo gelation after the polymer solution containing alginate and functionalized PEG contacts the crosslinker solution within the stomach (Fig. 1a,b). We extensively characterize LIFT hydrogels after ex vivo formation in real gastric fluid and in vivo formation in rodent and large porcine models, and demonstrate that their capacity to form solids in situ enables these materials to act as a depot for controlled release of small molecules. Moreover, co-encapsulation with CaCO3 as an excipient protects the activity of orally delivered enzymes and therapeutic bacteria from the low pH of the stomach (Fig. 1c).
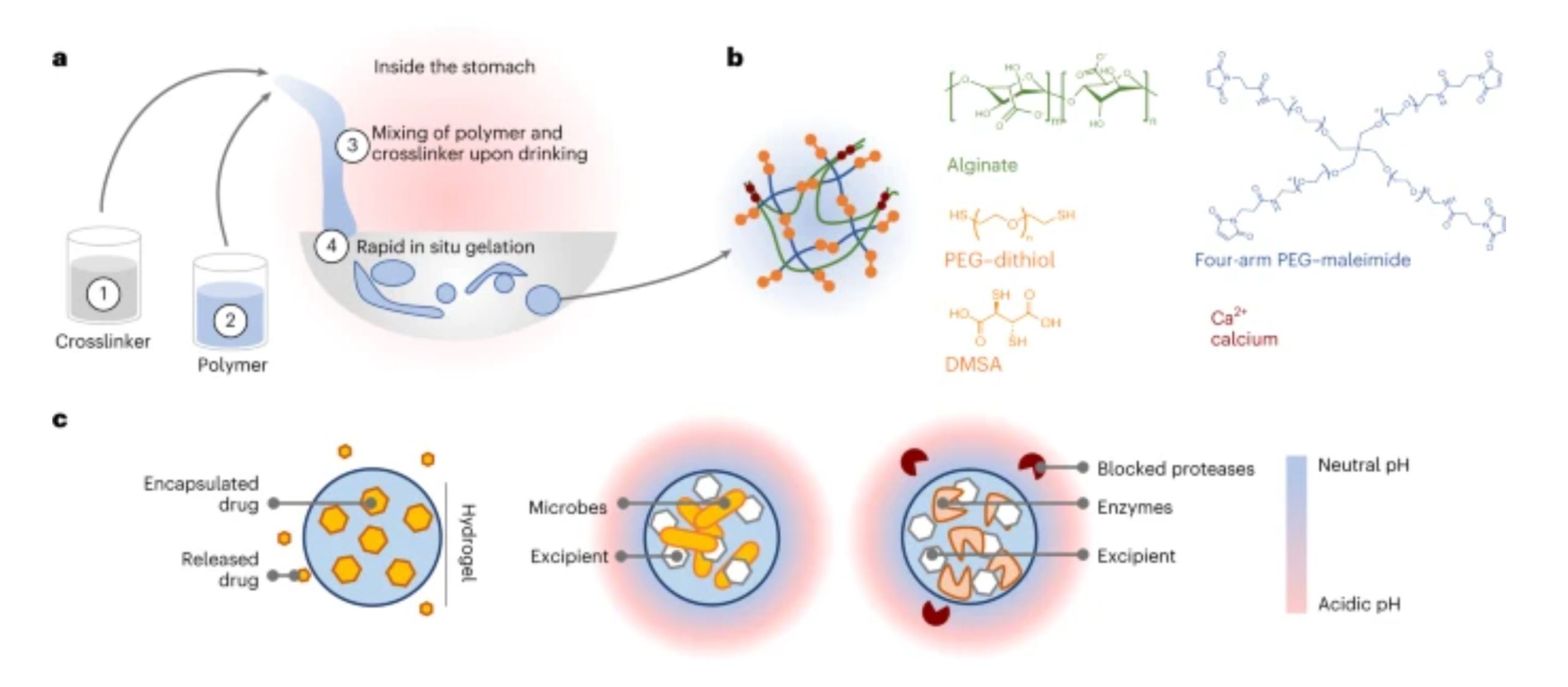
Fig. 1: Overview of LIFT hydrogels.
a, LIFT hydrogels form within the stomach after oral administration of (1) a 200-ml crosslinker solution comprising CaCl2 and a dithiol-containing molecule, followed by (2) a 20–40-ml polymer solution comprising alginate and four-arm PEG–maleimide. These two solutions (3) mix within the stomach to form a tough double-network hydrogel (4) within the stomach. b, Schematic of the polymers and reagents used to facilitate crosslinking. Materials were selected due to their established safety profiles. Both PEG–dithiol and dimercaptosuccinic acid (DMSA) were investigated as a dithiol crosslinker. c, Left: LIFT hydrogels may act as controlled release depots through encapsulation of water-insoluble drug that gradually dissolves and diffuses from the hydrogel. Middle and right: LIFT hydrogels enable co-encapsulation and co-localization of therapeutic microbes or enzymes and excipient (for example, CaCO3) that modulate local pH and protect against proteases.
Download the full article as PDF here Drinkable in situ-forming tough hydrogels for gastrointestinal therapeutics
or read it here
Liu, G.W., Pickett, M.J., Kuosmanen, J.L.P. et al. Drinkable in situ-forming tough hydrogels for gastrointestinal therapeutics. Nat. Mater. (2024). https://doi.org/10.1038/s41563-024-01811-5
Read also our introduction article on Alginates here:


