DuraLac® H – MEGGLE’s anhydrous lactose grade for direct compression

General information
Direct compression (DC) tablet manufacture is a popular choice because it provides the least complex, most cost effective process to produce tablets compared to other tablet manufacturing approaches. Manufacturers can blend APIs with excipients and compress, making dosage forms simple to produce [1, 2].
DC technology and the use of modern tableting equipment require that excipients and APIs form a compactible mixture with excellent flowability and low particle segregation tendency [3].
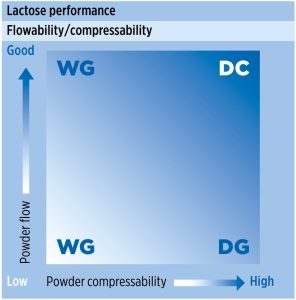
In the pharmaceutical industry, lactose is one of the most commonly used excipients; however, like many other excipients, lactose may not be suitable for direct compression without modification due to insufficient powder flow or/and compaction properties (figure 1).
Product description
DuraLac® H is produced by roller-drying a lactose solution at high temperature to form anhydrous beta-lactose and alpha-lactose crystals at levels approximating 80 % and 20 %, respectively. During anhydrous lactose crystallization, no water is incorporated in the crystal lattice [4].
Subsequent to roller-drying, anhydrous lactose is milled and sieved to the desired particle size distribution, optimizing powder flow and compactibility. DuraLac® H complies with the monograph „Lactose, anhydrous“ (Ph. Eur., USP-NF and JP). Because DuraLac® H deforms by brittle fracture during compaction, it is well suited for directly compressed and dry granulated formulations (roller compaction, slugging).
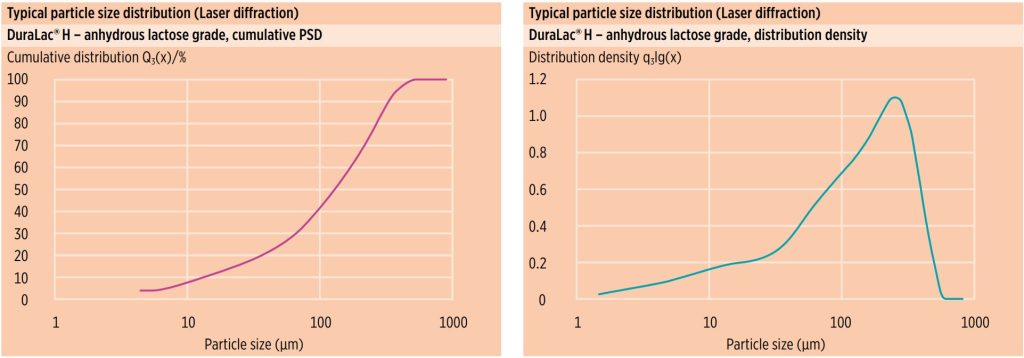
Particle size distribution (PSD)
Figure 2 shows typical laser diffraction particle size distribution data for MEGGLE’s anhydrous lactose grade, DuraLac® H.
Figure 3a and 3b depict specified PSD by air-jet sieving and Ro-Tap®. These parameters are also part of the in-process control (IPC).
Functional related characteristics
Powder flow
It is well-known that particle size and shape influence powder flowability. Particles smaller than 100 μm tend to be more cohesive and less freely flowing, whereas larger, denser particles tend to be more freely flowing. Particle morphology also significantly affects powder flow characteristics. Regarding flowability, figure 7 demonstrates that particle shape and structure are more important than the particle size distribution. Due to its shape, the anhydrous lactose flowability is moderate, but improves significantly with lubricant and/or glidant addition.
See the full brochure on “DuraLac®” here
(click the picture to download the brochure)
Benefits DuraLac®
- Excellent compactibility
- Good flowability
- Relatively low hygroscopicity (water sorption above 70 % relative humidity)
- High storage stability
- Excipient of choice for formulations requiring low water content
Source: MEGGLE brochure “DuraLac®”
See the overview video of the MEGGLE Dry Powder Inhalation product range here:
Company: MEGGLE is one of the world´s leading manufacturers of pharmaceutical grade lactose and co-processed excipients with expertise of more than 70 years. We encounter lactose in so many areas of our life – reason enough to take a closer look at this multi-functional “white powder”.
Do you need more information or a sample of DuraLac® excipients?


