Effect of Powdered Cellulose Nanofiber with Different Particle Sizes on the Physical Properties of Tablets Manufactured via Direct Compression
Abstract
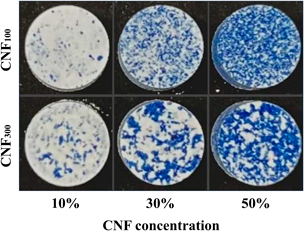
Direct compression is a tableting technique that involves a few steps in non-demanding manufacturing conditions. High strength and rapid disintegration of tablet formulations were previously achieved through the addition of cellulose nanofibers (CNFs), which have recently attracted attention as a high-performance biomass material. However, CNF addition results in greater variation in tablet weight and drug content, potentially due to differences in particle size between CNF and other additives. Herein, we used pulverized CNF to evaluate the effect of CNF particle size on the variation in tablet weight and drug content. Tablet formulations consisted of CNF with different particle sizes (approximately 100 µm [CNF100] and 300 µm [CNF300], at 0, 10, 30, or 50%), lactose hydrate, acetaminophen, and magnesium stearate. Ten powder formulations with different particle sizes and CNF concentrations were prepared; thereafter, the tablets were produced using a rotary tableting press with a compression force of 10 kN. The variation in weight and drug content as well as the tensile strength, friability, disintegration time, and drug dissolution of tablets were evaluated. CNF100 addition to the tablets reduced the weight and drug content variation to a greater extent than CNF300 addition. Using CNF300, we produced tablets of sufficient strength and short disintegration time. These properties were also achieved with CNF100 addition. Our findings suggest that adding CNF of small particle size to the tablet formulation can reduce the variation in weight and drug content while maintaining high strength and short disintegration time.
Introduction
Tablets are a convenient dosage form for patients as they are easy to carry and store, in addition to their accurate dosage. By coating the tablet, it is possible to mask the bitterness of the active ingredient and add various functionalities, such as sustained release, limited-time, and enteric coating.1) To produce tablets that meet clinical and market demands, pharmaceutical companies use high-speed tablet presses. Robust tablet quality should therefore be ensured and adapted to the various manufacturing environments.2–4)
The well-established technique of wet granule compression enables considerable tablet robustness.5–7) In recent years, direct compression, which requires fewer steps, has attracted increasing attention.8) In addition to the fewer steps required relative to wet granule compression, this technique can be performed in less-demanding manufacturing conditions. The reduced equipment, energy, and operating time required for the granulation process achieve a great reduction in cost and greater production efficiency. Therefore, direct compression has been the first choice for tablet manufacture in the United States in recent years.9) However, because the formulation powder, which is simply a mixture of the active ingredient and excipients, is placed into the tableting press and continuously compressed at a high speed, the weight and drug content of tablets tend to fluctuate greatly. To address these problems, in addition to examining formulation and manufacturing conditions, extensive research has focused on improving equipment and developing new additives.10–17)
In recent years, cellulose nanofibers (CNFs), which are made by refining cellulose, the main component of plant cell walls, have attracted attention in various fields as a high-performance biomass material.18) In Japan, the Ministry of the Environment is promoting a CNF performance evaluation model project aimed at reducing CO2 emissions, and naturally-derived CNFs extracted from plants are expected to contribute to the realization of a low-carbon society.19) Various companies and research institutes are currently participating in this project, with rigorous competition in development.
CNFs possess a large specific surface area owing to their fine fiber structure, in addition to remarkable properties, such as high strength, elasticity, and viscosity, while also being light in weight. Further, CNFs offer the advantage of being a renewable resource, with favorable characteristics such as biodegradability and biocompatibility. In the medical field, research on CNFs is underway for applications, such as wound treatment, scaffolding materials for bone grafting, artificial organs, and functional contact lenses.20,21) In the pharmaceutical industry, CNFs hold potential for improving drug delivery, release, and solubility, owing to their enormous specific surface area.22–28)
In direct compression, it is essential to increase the binding force between powder particles to form a tablet of sufficient strength. Therefore, microcrystalline cellulose (MCC), which is easily plastically deformed, has conventionally been used as a dry binder. Furthermore, disintegration of a tablet in water, which has a trade-off relationship with binding properties, affects not only the absorption and bioavailability of the drug, but also other characteristics of the formulation, such as rapid dissolution. To achieve the required disintegration characteristics, disintegrants with different disintegration capacities are selected separately from the binder and are added in combination in an optimal ratio. However, their inclusion as separate additives results in them constituting a certain proportion of the formulation. When a single additive can achieve both binding and disintegrating properties, it is not only advantageous when considering additive selection in formulation design, but also in determining the quality of the final product. Further, this approach is not complicated. Therefore, CNF, which increases the strength and disintegration of tablets to which it is added, is promising as a multifunctional additive with multiple functions. Previous studies have evaluated CNF as a novel additive with both binding and disintegrating functions.29,30) However, given the novelty of CNFs as materials and their limited exploration of applicability as pharmaceutical excipients, a comprehensive assessment of the effects of incorporating CNFs into the tablet formulation powder on the final product has been lacking.
In the present study, we focused on the strength and disintegration properties of CNF, evaluated the effect of CNF particle size on the physical properties of tablets manufactured via direct compression, and investigated the effectiveness of CNF as a multifunctional additive.
Download the full article as PDF here Effect of Powdered Cellulose Nanofiber with Different Particle Sizes on the Physical Properties of Tablets Manufactured via Direct Compression
or read it here
Materials
Lactose hydrate (Lac, diluent, Tablettose® 80, Meggle, Wasserburg, Germany), cellulose nanofiber (CNF, binder and disintegrant, ELLEX-P (alcohol-containing type), Daio Paper Corp., Tokyo, Japan), acetaminophen (APAP, model drug, Tyco Healthcare Japan, Tokyo, Japan), and magnesium stearate (Mg-St, lubricant, vegetable-based, FUJIFILM Wako Pure Chemical Corp., Osaka, Japan) were used to prepare the formulation powders. Commonly used additives, including microcrystalline cellulose (MCC; CEOLUS® KG-1000, Asahi Kasei Corp., Tokyo, Japan), low-substituted hydroxypropyl cellulose (L-HPC; LH-21; Shin-Etsu Chemical Co., Ltd., Tokyo, Japan), crospovidone (CP; Kollidon® CL, BASF, Ludwigshafen, Germany), and croscarmellose sodium (CCS; KICCOLATE™ ND-2HS, Asahi Kasei Corp.) were used as reference substances for each CNF.
Effect of Powdered Cellulose Nanofiber with Different Particle Sizes on the Physical Properties of Tablets Manufactured via Direct Compression, Shohei Nakamura, Mai Jinno, Momoka Hamaoka, Ayumi Sakurada and Takatoshi Sakamoto, Received August 29, 2023; accepted October 4, 2023
Read more on Magnesium Stearate as a pharmaceutical excipient here:


