Mineral Salts Low in Endotoxins for parenteral Medicines
Low in Endotoxin Products
Endotoxins are lipopolysaccharides (LPS) which are outer membrane components of gram-negative bacteria. By decomposing, these bacteria release lipopolysaccharides, which are toxic.
Parenteral medicines such as solutions for dialysis, hemofiltration and osmotherapy constitute a marked quality standard. Systemic parenteral application of infusions or injections with endotoxin quantities as low as 0.1 ng per kilogram of bodyweight can cause various physiological reactions, including fever, inflammation and sickness behavior in humans and animals. Due to the high sensitive application form, the manufacture of parenteral medicines requires particular caution: it is subject to strict and specific regulatory requirements and is regulated by Chapter 2.6.14 of the European Pharmacopoeia (Ph.Eur.). In order to support manufacturers of parenteral products to comply with legal and quality requirements, Dr. Paul Lohmann® offers Mineral Salts with a very low content of endotoxins.
Within the biotech industry, Low in Endotoxin qualities are of special interest for cell cultures. Endotoxins can cause undesirable effects on cell proliferation or cell function. Due to the high purity of our products, highly sensitive cell lines can develop in an optimal and laboratory controlled environment.
Low in Endotoxin Qualities of Dr. Paul Lohmann® Products
In 2017, a purpose-built plant was brought into service. This plant is GMP-certified and solely dedicated for the production of low endotoxin qualities. Thus our production process of high purity mineral salts including the use of low endotoxin process water is especially conceptualized for the purpose of the production of parenteral medicines.
- LAL test* according to Ph.Eur. is performed on every single batch.
- Our customers can optimally integrate our low in endotoxin qualities in their process chain for the manufacture of parenteral dosage forms (please see blue text on the next page).
Low in Endotoxin mineral compounds can be used for the production of the following products:
- Solutions for parenterals:
- Injections
- Infusions
- Nutrition
- Solutions for inhalants
- Solutions for
- Dialysis
- Peritoneal dialysis
- Haemofiltration
- Osmotherapy
- Ophthalmic preparations
- Biopharmaceuticals
Pharmaceutical manufacturers of preparations for infusion or injection are obliged to observe special regulations specified by the relevant pharmacopoeia and good manufacturing practices.
We emphasize that our products are low in endotoxin content but not entirely free of endotoxins and not sterile.
Therefore, these products must be treated with special procedures prior to their use in finished pharmaceutical products.
The pharmaceutical manufacturer must ensure that our
products undergo special procedures and tests prior to being processed for infusion or injection preparations in order to induce their suitability for their intended purpose.
Products in Low in Endotoxin Quality
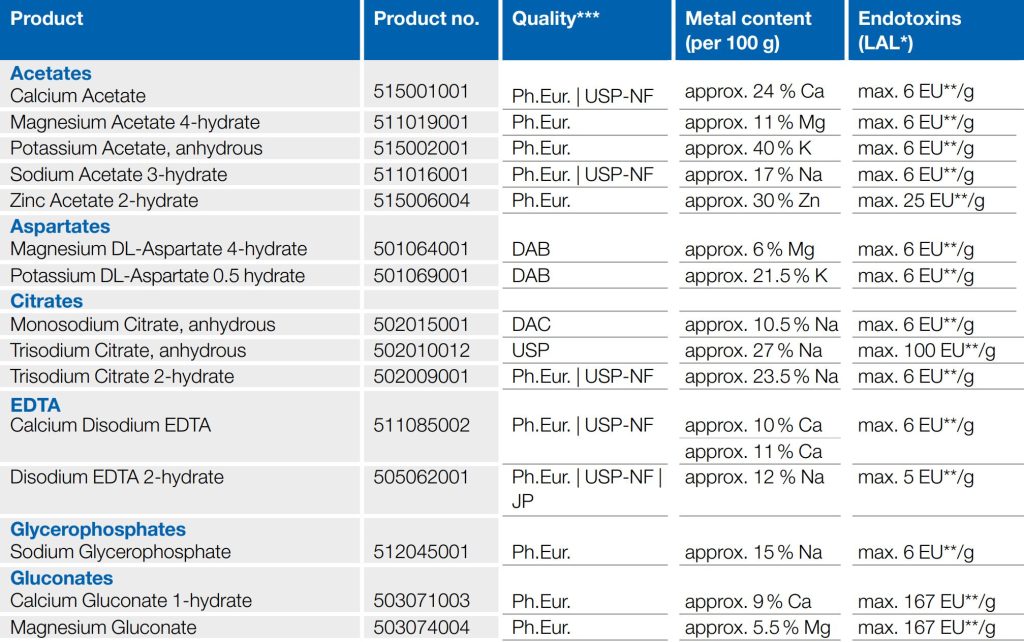
Additional products can be developed or lower endotoxin limits (LAL) can be met upon customer request.
See the full brochure on “Mineral Salts Low in Endotoxins” here
(click the picture to download the brochure)
Source: Dr. Paul Lohmann, full brochure “Mineral Salts Low in Endotoxins“
Do you need more information or a sample of Dr. Paul Lohmann excipients?