A systematic approach for assessing the suitability of enteral feeding tubes for the administration of controlled-release pellet formulations
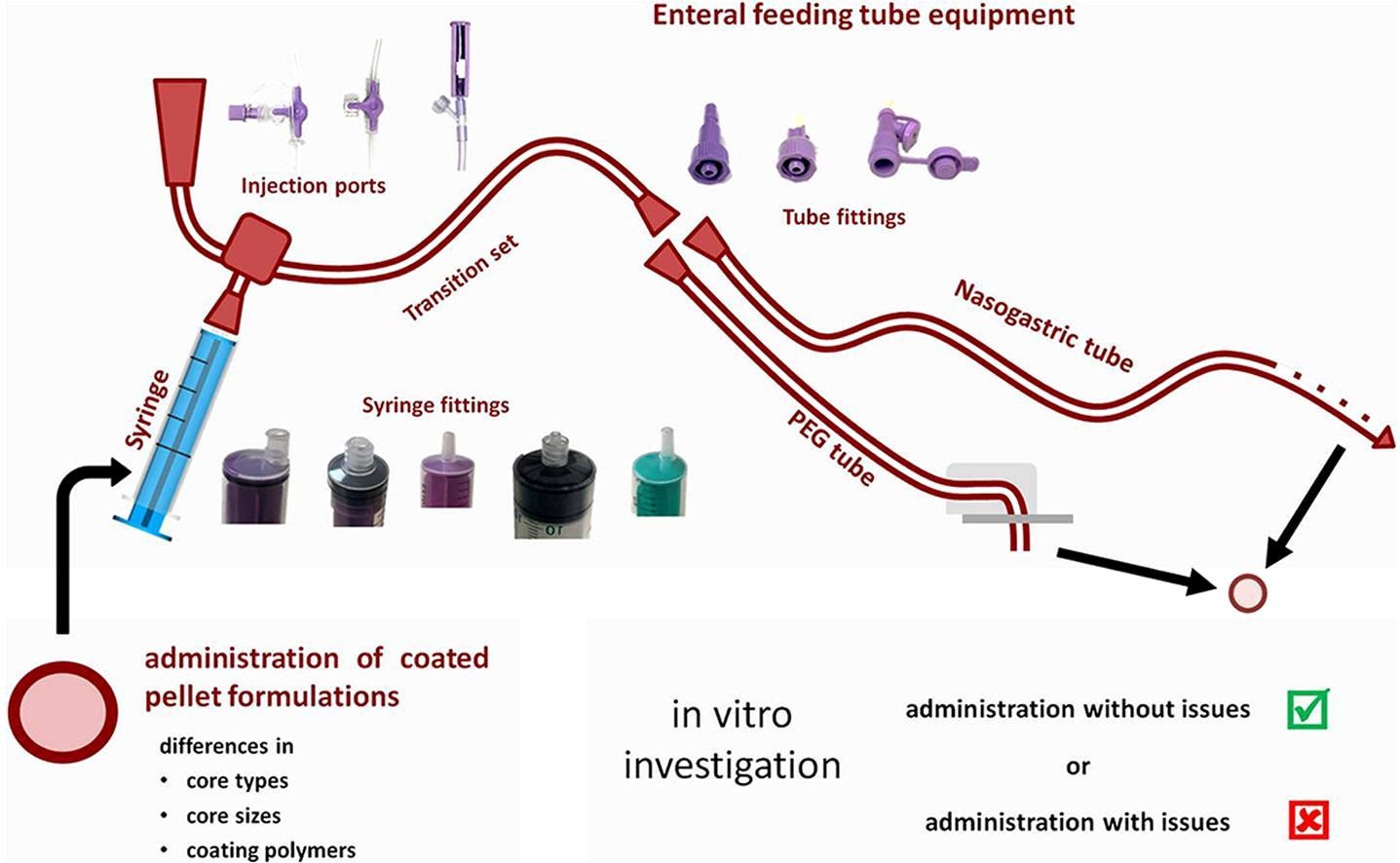
Enteral nutrition plays an important role for patients who are unable to properly swallow food. In such patients, enteral feeding tubes are often used, through which food, but often also oral medications, are administered. However, this can pose the risk of tube clogging. Compared to the administration of crushed tablets, multiparticulate dosage forms are often considered easier to administer and furthermore have the advantage of enabling the administration of even controlled-release preparations.
The objective of this systematic study was to identify tube- and formulation-related factors that contribute to successful administration of coated pellet formulations via a variety of commercially available feeding tube devices. The suitability of enteral feeding tubes for the administration of controlled-release pellet formulations that differed in size and type of starter core and functional coating was investigated in a stepwise approach using a novel in vitro setup. Results of the study indicate that pellet diameter and inner diameter of the feeding tube are by no means reliable parameters for estimating the tube’s suitability for pellet administration, but that many other tube and formulation-related factors and combinations thereof must be considered to ensure safe and effective drug administration via enteral feeding tubes.
Frank Karkossa, Nicole Lehmann, Sandra Klein,
A systematic approach for assessing the suitability of enteral feeding tubes for the administration of controlled-release pellet formulations,
International Journal of Pharmaceutics, Volume 612, 2022, 121286, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2021.121286.
Materials
Neutral microcrystalline cellulose (MCC) pellets (Cellets® 500–710 µm, # 16L1067 and Cellets® 1000–1500 µm, # 17H1019), hereafter referred to as Cellets 500 or 1000, respectively, were kindly provided by Harke Pharma GmbH, Mühlheim, Germany. Neutral pellets made of sucrose and maize starch (Sugar Spheres® 500–600 µm, # 08130075 and Sugar Spheres® 1180–1400 µm, # 08630011), hereafter named as Sugar Spheres 500 and 1000, were a gift from Hanns G. Werner GmbH Co. KG, Tornesch, Germany. The polymethacrylate-based coating dispersions, methacrylic acid – ethylacrylate copolymer (1:1) (MAE, Eudragit® L 30 D-55 (# C181014657)), ammonio methacrylate copolymer (AmM) type A (Eudragit® RL 30 D, # G180616589) and type B (Eudragit® RS 30 D, # G180618588) were provided by Evonik Nutrition & Care GmbH, Essen, Germany. Polyvinyl acetate (PVAc, Kollicoat® SR 30 D) and polyvinylpyrrolidon (PVP, Kollidon® 30) were donated by BASF SE, Ludwigshafen, Germany. Ethyl cellulose (EC) dispersion (Surelease®, # IN539100) was provided by Colorcon Indianapolis, USA. Micronized talc (ECSA Chemicals AG, Flawil, Switzerland) and triethyl citrate (Merck, Darmstadt, Germany) were purchased commercially. All other reagents used in the study were of analytical grade and purchased commercially. Enteral feeding tubes, syringes, connectors and transition sets were either provided by the manufacturers or purchased commercially. Details on these materials are provided in the following sections.
See more on pharm-a-spheres sugar spheres: