Batch versus continuous blending of binary and ternary pharmaceutical powder mixtures
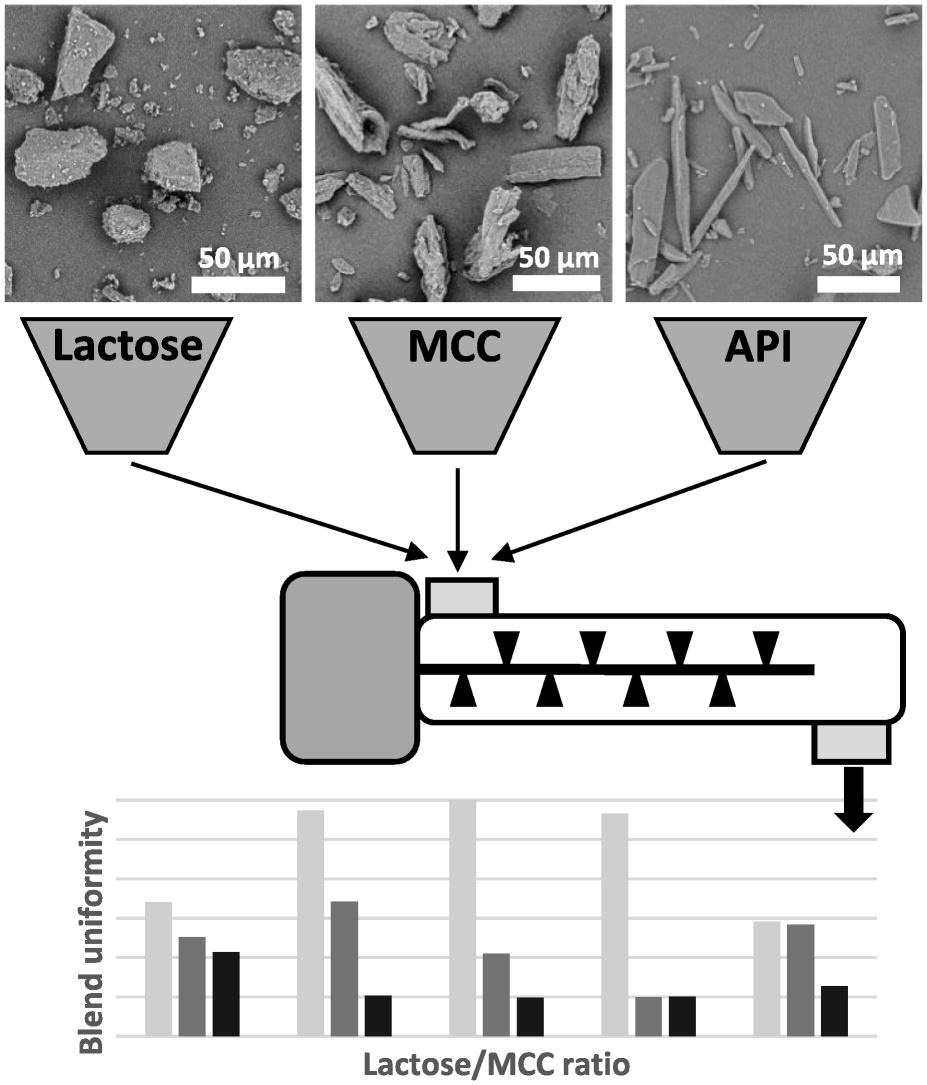
The material properties of excipients and active pharmaceutical ingredients (API’s) are important parameters that affect blend uniformity of pharmaceutical powder formulations. With the current shift from batch to continuous manufacturing in the pharmaceutical industry, blending of excipients and API is converted to a continuous process. The relation between material properties and blend homogeneity, however, is generally based on batch-wise blending trials. Limited information is available on how material properties affect blending performance in a continuous process. Here, blending of API and excipients is studied in both a batch and a continuous process. Homogeneity of the resulting mixtures is analyzed, which reveals that the impact of material properties is very different in a continuous process. Where parameters such as particle size, density and flowability have significant impact on blending performance in a traditional batch process, continuous blending is more robust resulting in uniform blends for a large variety of blend compositions.
Download the research paper as PDF: Batch versus continuous blending of binary and ternary pharmaceutical powder mixtures
About the article: Maarten Jaspers, Sri Sharath Kulkarni, Florian Tegel, Timo P. Roelofs, Myrthe T.W. de Wit, Pauline H.M. Janssen, Bernhard Meir, Ralf Weinekötter, Bastiaan H.J. Dickhoff, Batch versus continuous blending of binary and ternary pharmaceutical powder mixtures, International Journal of Pharmaceutics: X, Volume 4, 2022, 100111, ISSN 2590-1567, https://doi.org/10.1016/j.ijpx.2021.100111 (https://www.sciencedirect.com/science/article/pii/S2590156721000402)
Materials
Anhydrous lactose (SuperTab® 21AN), milled lactose monohydrate (Pharmatose® 200M), spray dried lactose (SuperTab® 11SD), agglomerated lactose (SuperTab® 30GR) and microcrystalline cellulose (Pharmacel® 101 and Pharmacel® 102) were obtained from DFE Pharma (Goch, Germany). Paracetamol standard powder and dense powder were purchased from Mallinckrodt Inc. (Raleigh, NC, USA) and are used as a model drug in the current study. The paracetamol standard powder was sieved using an oscillatory sieve with a 315 μm mesh before use to remove large agglomerates.

